
Chinese Journal OF Rice Science ›› 2022, Vol. 36 ›› Issue (4): 357-366.DOI: 10.16819/j.1001-7216.2022.210711
• Research Papers • Previous Articles Next Articles
SUN Zhiguang1, DAI Huimin2, CHEN Tingmu1, LI Jingfang1, CHI Ming1, ZHOU Zhenling1, LIU Yan1, LIU Jinbo1, XU Bo1, XING Yungao1, YANG Bo1, LI Jian1, LU Baiguan1, FANG Zhaowei1, WANG Baoxiang1,*( ), XU Dayong1,*(
), XU Dayong1,*( )
)
Received:2021-07-30
Revised:2021-08-30
Online:2022-07-10
Published:2022-07-12
Contact:
WANG Baoxiang, XU Dayong
孙志广1, 代慧敏2, 陈庭木1, 李景芳1, 迟铭1, 周振玲1, 刘艳1, 刘金波1, 徐波1, 邢运高1, 杨波1, 李健1, 卢百关1, 方兆伟1, 王宝祥1,*( ), 徐大勇1,*(
), 徐大勇1,*( )
)
通讯作者:
王宝祥,徐大勇
基金资助:SUN Zhiguang, DAI Huimin, CHEN Tingmu, LI Jingfang, CHI Ming, ZHOU Zhenling, LIU Yan, LIU Jinbo, XU Bo, XING Yungao, YANG Bo, LI Jian, LU Baiguan, FANG Zhaowei, WANG Baoxiang, XU Dayong. Phenotypic Identification and Gene Mapping of a Lesion Mimic Mutant lmm7 in Rice[J]. Chinese Journal OF Rice Science, 2022, 36(4): 357-366.
孙志广, 代慧敏, 陈庭木, 李景芳, 迟铭, 周振玲, 刘艳, 刘金波, 徐波, 邢运高, 杨波, 李健, 卢百关, 方兆伟, 王宝祥, 徐大勇. 水稻类病斑突变体lmm7的鉴定与基因定位[J]. 中国水稻科学, 2022, 36(4): 357-366.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2022.210711
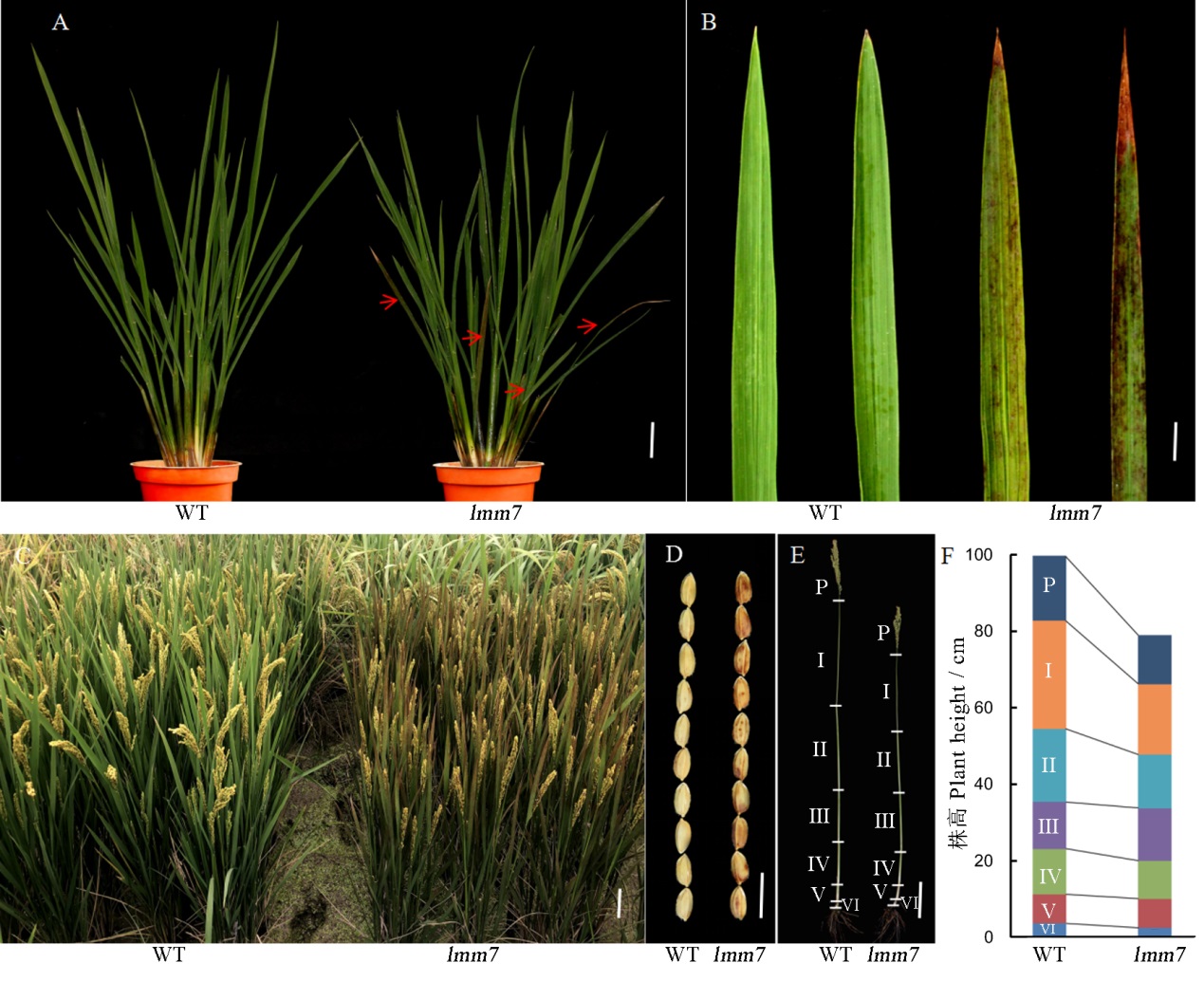
Fig. 1. Phenotypic identification of WT plants and lmm7. A, Plants of wild type (WT) and lmm7 mutant at the tillering stage, red arrows indicate lesion mimic leaves; B, Basal leaves of wild type (WT) and lmm7 at the tillering stage; C, Plants of wild type (WT) and lmm7 at the mature period in field conditions; D, Comparisons of the size of mature seeds from WT and lmm7 plants; E-F: Panicles and internodes of main culms. P, Panicle; I to VI indicate corresponding internodes from top to bottom. Bars =5 cm in A; 2 cm in B; 10 cm in C, E; 10 mm in D.
| 性状 Trait | 野生型Wild type | 突变体 lmm7 | 性状 Trait | 野生型Wild type | 突变体 lmm7 |
|---|---|---|---|---|---|
| 抽穗期 Heading date /d | 95.3±0.6 | 95.7±0.6 | 千粒重 1000-grain weight/g | 24.6±0.4 | 23.8±0.5 |
| 株高 Plant height /cm | 102.1±2.6 | 80.8±1.8** | 剑叶长 Flag leaf length/cm | 24.5±1.4 | 18.8±1.2** |
| 穗长 Panicle length /cm | 17.4±0.8 | 13.0±1.2** | 剑叶宽 Flag leaf width/cm | 1.8±0.3 | 1.6±0.2** |
| 有效穗数 Effective panicle | 9.2±1.3 | 6.5±0.7* | 粒长 Grain length/mm | 7.6±0.4 | 7.5±0.6 |
| 每穗粒数 Grains per panicle | 142.3±6.5 | 115.4±7.6** | 粒宽 Grain width /mm | 4.4±0.2 | 4.3±0.1 |
| 结实率 Seed setting rate /% | 90.6±2.5 | 82.7±4.3* | 粒厚 Grain thickness /mm | 2.1±0.2 | 2.1±0.2 |
Table 1. Agronomic traits comparison between WT plants and lmm7.
| 性状 Trait | 野生型Wild type | 突变体 lmm7 | 性状 Trait | 野生型Wild type | 突变体 lmm7 |
|---|---|---|---|---|---|
| 抽穗期 Heading date /d | 95.3±0.6 | 95.7±0.6 | 千粒重 1000-grain weight/g | 24.6±0.4 | 23.8±0.5 |
| 株高 Plant height /cm | 102.1±2.6 | 80.8±1.8** | 剑叶长 Flag leaf length/cm | 24.5±1.4 | 18.8±1.2** |
| 穗长 Panicle length /cm | 17.4±0.8 | 13.0±1.2** | 剑叶宽 Flag leaf width/cm | 1.8±0.3 | 1.6±0.2** |
| 有效穗数 Effective panicle | 9.2±1.3 | 6.5±0.7* | 粒长 Grain length/mm | 7.6±0.4 | 7.5±0.6 |
| 每穗粒数 Grains per panicle | 142.3±6.5 | 115.4±7.6** | 粒宽 Grain width /mm | 4.4±0.2 | 4.3±0.1 |
| 结实率 Seed setting rate /% | 90.6±2.5 | 82.7±4.3* | 粒厚 Grain thickness /mm | 2.1±0.2 | 2.1±0.2 |
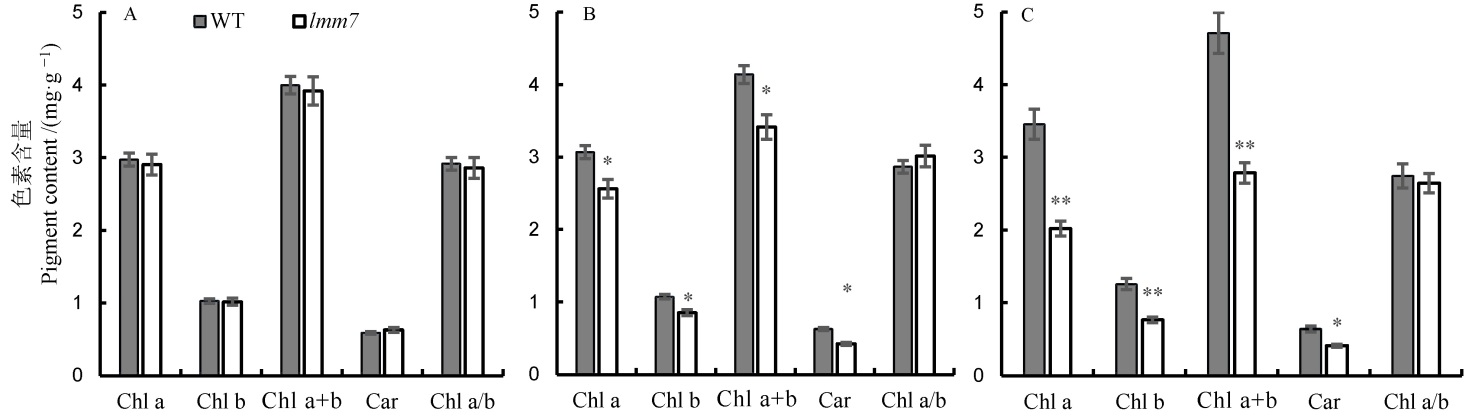
Fig. 2. Photosynthetic pigments contents of the wild type and the lmm7 mutant at heading stage. A, Photosynthetic pigment contents of flag leaf of the wild type and lmm7 mutant at the heading stage; B, Photosynthetic pigment contents of the second leaf from top; C, Photosynthetic pigment contents of the third leaf from top; *,** significantly different at 0.05 and 0.01 levels, respectively.
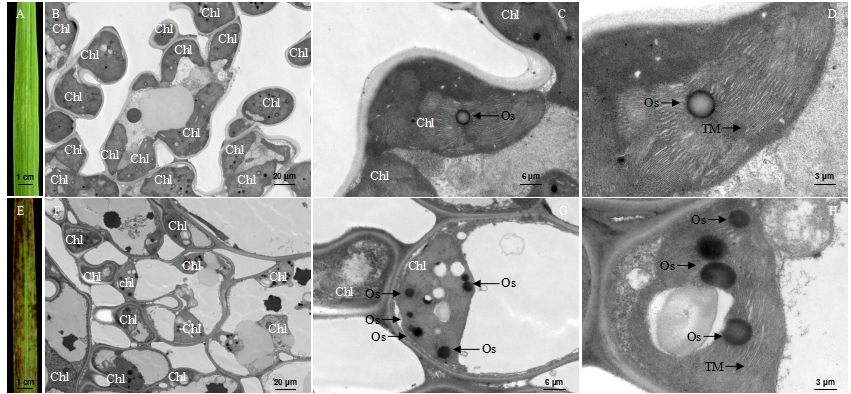
Fig. 3. Ultrastructure of mesophyll cells in the wild type (WT) and the lmm7 mutant at mature period. A, Flag leaf of WT at mature period; B-D, Ultrastructure of the blade of WT; E, Flag leaf of lmm7 mutant at mature period; F-G, Ultrastructure of the blade of lmm7 mutant; Chl, Chloroplast; Os, Osmiophilic granule; TM, Thylakoid membranes. Bars: 20 μm (B, F), 6 μm (C, G), 3 μm (D, H).

Fig. 4. Shading treatment and histochemical analysis of the wild type and lmm7 mutant. A, Effects of shading on the wild type(WT) and the lmm7 mutant leaves 7 days after shading; Leaves of WT and the lmm7 mutant stained by trypan blue(B) and DAB(C). Bars =1 cm in A, B and C.
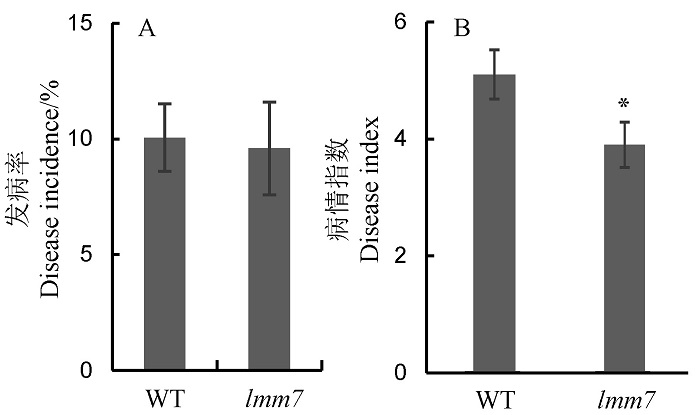
Fig. 5. Resistance evaluation of wild type (WT) and lmm7 mutant to rice black-streaked dwarf virus disease (RBSDVD) and rice blast under artificial inoculation condition. A, Disease incidence of wild type (WT) and lmm7 mutant to rice black-streaked dwarf virus disease (RBSDVD) by the artificial inoculation. B, Disease index of wild type (WT) and lmm7 to rice blast by the artificial inoculation. *P < 0.05 by Student’s t-test.
| [1] | 王晨, 王备芳, 张迎信, 曹永润, 张越, 江敏, 边康吉, 张小惠, 刘群恩. 水稻类病斑突变体lm8015-2的鉴定与基因的精细定位[J]. 中国水稻科学, 2021, 35(4): 352-358. |
| Wang C, Wang B F, Zhang Y X, Cao Y R, Zhang Y, Jiang M, Bian K J, Zhang X H, Liu Q E. Identification and gene mapping of a lesion mimic mutant lm8015-2 in rice[J]. Chinese Journal of Rice Science, 2021, 35(4): 352-358. (in Chinese with English abstract) | |
| [2] | Hu G, Richter T E, Hulbert S H, Pryor T. Disease lesion mimicry caused mutations in the rust resistance gene rp1[J]. Plant Cell, 1996, 8(8): 1367-1376. |
| [3] | Wu C J, Bordeos A, Madamba M R S, Baraoidan M, Ramos M, Wang G L, Leach J E, Leung H. Rice lesion mimic mutants with enhanced resistance to diseases[J]. Molecular Genetics and Genomics, 2008, 279: 605-619. |
| [4] | Shirsekar G S, Vega-Sanchez M E, Bordeos A, Baraoidan M, Swisshelm A, Fan J, Park C H, Leung H, Wang G L. Identification and characterization of suppressor mutants of spl11-mediated cell death in rice[J]. Molecular Plant-Microbe Interactions, 2014, 27: 528-536. |
| [5] | Wang S, Lei C L, Wang J L, Ma J, Tang S, Wang C L, Zhao K J, Tian P, Zhang H, Qi C Y, Cheng Z J, Zhang X, Guo X P, Liu L L, Wu C Y, Wan J M. SPL33, encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice[J]. Journal of Experimental Botany, 2017, 68: 899-913. |
| [6] | Hofius D, Tsitsigiannis D I, Jones J D, Mundy J. Inducible cell death in plant immunity[J]. Seminars in Cancer Biology, 2007, 17: 166-187. |
| [7] | Dietrich R A, Richberg M H, Schmidt R, Dean C, Dangl J L. A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death[J]. Cell, 1997, 88: 685-694. |
| [8] | Hoisington D A, Neuffer M G, Walbot V. Disease lesion mimics in maize: I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1[J]. Developmental Biology, 1982, 93(2): 381-388. |
| [9] | Yao Q, Zhou R H, Fu T H, Wu W R, Zhu Z D, Li A L, Jia J Z. Characterization and mapping of complementary lesion-mimic genes lm1 and lm2 in common wheat[J]. Theoretical and Applied Genetics, 2009, 119: 1005-1012. |
| [10] | Spassieva S, Hille J. A lesion mimic phenotype in tomato obtained by isolating and silencing an Lls1, homologue[J]. Plant Science, 2002, 162: 543-549. |
| [11] | Wolter M, Hollricher K, Salamini F, Schulze-Lefert P. The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defence mimic phenotype[J]. Molecular and General Genetics, 1993, 239: 122-128. |
| [12] | 张宏根, 王茂宇, 张丽佳, 胡雅, 马佳琦, 张翼帆, 汤述翥, 梁国华, 顾铭洪. 水稻类病斑突变体wy3的鉴定和基因定位[J]. 中国水稻科学, 2016, 30(3): 239-246. |
| Zhang H G, Wang M Y, Zhang L J, Hu Y, Ma J Q, Zhang Y F, Tang S Z, Liang G H, Gu M H. Characterization and gene mapping of lesion mimic mutant wy3 in rice[J]. Chinese Journal of Rice Science, 2016, 30(3): 239-246. (in Chinese with English abstract) | |
| [13] | Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, Daelen R, Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P. The barley mlo gene: A novel control element of plant pathogen resistance[J]. Cell, 1997, 88: 695-705. |
| [14] | Jambunathan N, Siani J M, Mcnellis T W. A humidity sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance[J]. Plant Cell, 2001, 13: 2225-2240. |
| [15] | Lorrain S, Lin B, Auriac M C, Kroj T, Saindrenan P, Nicole M, Balague C, Roby D. Vascular associated death1, a novel GRAM domain-containing protein, is a regulator of cell death and defense responses in vascular tissues[J]. Plant Cell, 2004, 16: 2217-2232. |
| [16] | Noutoshi Y, Kuromori T, Wada T, Hirayama T, Kamiya A, Imura Y, Yasuda M, Nakashita H, Shirasu K, Shinozaki K. Loss of Necrotic Spotted Lesions 1 associates with cell death and defense responses in Arabidopsis thaliana[J]. Plant Molecular Biology, 2006, 62: 29-42. |
| [17] | Balague C, Lin B, Alcon C, Flottes G, Malmstrom S, Kohler C, Neuhaus G, Pelletier G, Gaymard F, Roby D. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family[J]. Plant Cell, 2003, 15: 365-379. |
| [18] | Rostoks N, Schmierer D, Mudie S, Drader T, Brueggeman R, Caldwell D G, Waugh R, Kleinhofs A. Barley necrotic locus nec1 encodes the cyclic nucleotide-gated ion channel 4 homologous to the Arabidopsis HLM1[J]. Molecular Genetics and Genomics, 2006, 275: 159-168. |
| [19] | Mosher S, Moeder W, Nishimura N, Jikumaru Y, Joo S H, Urquhart W, Klessig D F, Kim S K, Nambara E, Yoshioka K. The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner[J]. Plant Physiology, 2010, 152: 1901-1913. |
| [20] | Wang L, Pei Z, Tian Y, He C. OsLSD1, a rice zinc finger protein, regulates programmed cell death and callus differentiation[J]. Molecular Plant Microbe Interactions, 2005, 18: 375-384. |
| [21] | Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein[J]. Proceedings of National Academy of Sciences of the USA, 2002, 99: 7530-7535. |
| [22] | Zeng L, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm B H, Leung H, Wang G. Spotted leaf 11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity[J]. Plant Cell, 2004, 16: 2795-2808. |
| [23] | Qiao Y, Jiang W, Lee J, Park B, Choi M S, Piao R, Woo M O, Roh J H, Han L, Paek N C, Seo H S, Koh H J. SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa)[J]. New Phytologist, 2010, 185: 258-274. |
| [24] | Kachroo P, Shanklin J, Shah J, Whittle E J, Klessig D F. A fatty acid desaturase modulates the activation of defense signaling pathways in plants[J]. Proceedings of National Academy of Sciences of the USA, 2001, 98: 9448-9453. |
| [25] | Brodersen P, Petersen M, Pike H M, Olszak B, Skov S, Odum N, Jorgensen L B, Brown R E, Mundy J. Knockout of Arabidopsis accelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense[J]. Genes & Development, 2002, 16: 490-502. |
| [26] | Hu G, Yalpani N, Briggs S P, Johal G S. A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize[J]. Plant Cell, 1998, 10: 1095-1105. |
| [27] | Ishikawa A, Okamoto H, Iwasaki Y, Asahi T. A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis[J]. Plant Journal, 2001, 27: 89-99. |
| [28] | Gray J, Close P S, Briggs S P, Johal G S. A novel suppressor of cell death in plants encoded by the Lls1 gene of maize[J]. Cell, 1997, 89: 25-31. |
| [29] | Undan J R, Tamiru M, Abe A, Yoshida K, Kosugi S, Takagi H, Yoshida K, Kanzaki H, Saitoh H, Fekih R. Mutation in OsLMS, a gene encoding a protein with two double-stranded RNA binding motifs causes lesion mimic phenotype and early senescence in rice (Oryza sativa L.)[J]. Genes & Genetic Systems, 2012, 87(3): 169-179. |
| [30] | Wang Z, Wang Y, Hong X, Hu D, Liu C, Yang J, Li Y, Huang Q, Feng Y, Gong H, Li Y, Fang G, Tang H, Li Y,. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice[J]. Journal of Experimental Botany, 2015, 66: 973-987. |
| [31] | Lichtenthaler H K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes[J]. Methods in Enzymology, 1987, 148: 350-382. |
| [32] | Fang L K, Li Y F, Gong X P, Sang X C, Ling Y H, Wang X W, Cong Y F, He G H. Genetic analysis and gene mapping of a dominant presenescing leaf gene PSL3 in rice (Oryza sativa L.)[J]. Chinese Science Bulletin, 2010, 55: 2517-2521. |
| [33] | 刘永锋, 陆凡, 陈志谊, 吉建安, 陈毓苓, 高渊. 江苏省水稻新品种(系)对稻瘟病的抗性评价[J]. 中国水稻科学, 2002, 16(1): 96-98. |
| Liu Y F, Lu G, Chen Z Y, Ji J A, Chen Y L, Gao Y. Resistance evaluation of new rice varieties (lines) to rice blast in Jiangsu Province[J]. Chinese Journal of Rice Sciences, 2002, 16(1): 96-98. | |
| [34] | International Rice Research Institute. Standard Evaluation System for Rice (SES). Los Baños, Philippines: International Rice Research Institute, 2002: 14-18. |
| [35] | Zhou T, Du L, Wang L J, Wang Y, Gao C Y, Lan Y, Sun F, Fan Y J, Wang G L, Zhou Y J. Genetic analysis and molecular mapping of QTLs for resistance to rice black-streaked dwarf disease in rice[J]. Scientific Reports, 2015, 5: 10509. |
| [36] | Zhu X B, Ze M, Chen M S, Chen X W, Wang J. Deciphering rice lesion mimic mutants to understand molecular network governing plant immunity and growth[J]. Rice Science, 2020, 27(4): 278-288. |
| [37] | 奉保华, 杨杨, 施勇烽, 林璐, 陈洁, 黄奇娜, 魏彦林, LEUNG H, 吴建利. 水稻淡褐斑叶突变体lbsl1的遗传分析与基因定位[J]. 中国水稻科学, 2012, 26(3): 297-301. |
| Feng B H, Yang Y, Shi Y F, Lin L, Chen J, Huang Q N, Wei Y L, Leung H, Wu J L. Genetic analysis and gene mapping of a light brown spotted leaf mutant in rice[J]. Chinese Journal of Rice Sciences, 2012, 26(3): 297-301. (in Chinese with English abstract) | |
| [38] | 王建军, 朱旭东, 王林友, 张利华, 薛庆中, 何祖华. 水稻类病斑突变体的生理与遗传分析[J]. 植物生理与分子生物学学报, 2004, 30(3): 331-338. |
| Wang J J, Zhu X D, Wang L Y, Zhang L H, Xue Q Z, He Z H. Physiological and genetic analysis of lesion resembling disease mutants (lrd) of Oryza sativa L[J]. Journal of Plant Physiology and Molecular Biology, 2004, 30(3): 331-338. (in Chinese with English abstract) | |
| [39] | Yoshimura A, Ideta O, Iwata N. Linkage map of phenotype and RFLP markers in rice[J]. Plant Molecular Biology, 1997, 35: 49-60. |
| [40] | Chern M, Xu Q F, Bart R S, Bai W, Ruan D L, Sze-To W H, Canlas P E, Jain R, Chen X W, Ronald P C. A genetic screen identifies a requirement for cysteine-rich- receptor-like kinases in rice NH1 (OsNPR1)-mediated immunity[J]. PLoS Genetics, 2016, 12(5): e1006049. |
| [41] | Tang J Y, Zhu X D, Wang Y Q, Liu L C, Xu B, Li F, Fang J, Chu C C. Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice[J]. Plant Journal, 2011, 66: 996-1007. |
| [42] | Li Z Q, Ding B, Zhou X P, Wang G L. The rice dynamin related protein OsDRP1E negatively regulates programmed cell death by controlling the release of cytochrome c from Mitochondria[J]. PLoS Pathogens, 2017, 13(1): e1006157. |
| [43] | Chen X F, Pan J W, Cheng J, Jiang G H, Jin Y, Gu Z M, Qian Q, Zhai W X, Ma B J. Fine genetic mapping and physical delimitation of the lesion mimic gene spotted leaf 5 (spl5) in rice (Oryza sativa L.)[J]. Molecular Breeding, 2009, 24(4): 387. |
| [44] | Chen X F, Hao L, Pan J W, Zheng X X, Jiang G H, Jin Y, Gu Z M, Qian Q, Zhai W X, Ma B J. SPL5, a cell death and defense related gene, encodes a putative splicing factor 3b subunit 3 (SF3b3) in rice[J]. Molecular Breeding, 2012, 30(2): 939-949. |
| [45] | Babu R, Jiang C J, Xu X, Kottapalli K R, Takatsuji H, Miyao A, Hirochika H, Kawasaki S. Isolation, fine mapping and expression profiling of a lesion mimic genotype, spl(NF4050-8) that confers blast resistance in rice[J]. Theoretical and Applied Genetics, 2011, 122: 831-854. |
| [46] | Li Z, Zhang Y, Liu L, Liu Q, Bi Z, Yu N, Cheng S, Cao L. Fine mapping of the lesion mimic and early senescence 1 (lmes1) in rice (Oryza sativa)[J]. Plant Physiology and Biochemistry, 2014, 80: 300-307. |
| [47] | 夏赛赛, 崔玉, 李凤菲, 谭佳, 谢园华, 桑贤春, 凌英华. 水稻类病斑早衰突变体lmps1的表型鉴定与基因定位[J]. 作物学报, 2019, 45(1): 46-54. |
| Xia S S, Cui Y, Li F F, Tan J, Xie Y H, Sang X C, Ling Y H. Phenotypic characterizing and gene mapping of a lesion mimic and premature senescence 1 (lmps1) mutant in rice (Oryza sativa L.)[J]. Acta Agronomica Sinica, 2019, 45(1): 46-54. (in Chinese with English abstract) | |
| [48] | 郭丹, 施勇烽, 王惠梅, 张晓波, 宋莉欣, 徐霞, 贺彦, 郭梁, 吴建利. 一个水稻显性斑点叶突变体的鉴定和基因精细定位[J]. 作物学报, 2016, 42(7): 966-975. |
| Guo D, Shi Y F, Wang H M, Zhang X B, Song L X, Xu X, Guo L, Wu J L. Characterization and gene fine mapping of a rice dominant spotted-leaf mutant[J]. Acta Agronomica Sinica, 2016, 42(7): 966-975. (in Chinese with English abstract) | |
| [49] | Sun L T, Wang Y H, Liu L L, Wang C M, Gan T, Zhang Z Y, Wang Y L, Wang D, Niu M, Long W H, Li X H, Zheng M, Jiang L, Wan J M. Isolation and characterization of a spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice[J]. Scientific Reports, 2017, 7: 41846. |
| [1] | HOU Xiaoqin, WANG Ying, YU Bei, FU Weimeng, FENG Baohua, SHEN Yichao, XIE Hangjun, WANG Huanran, XU Yongqiang, WU Zhihai, WANG Jianjun, TAO Longxing, FU Guanfu. Mechanisms Behind the Role of Potassium Fulvic Acid in Enhancing Salt Tolerance in Rice Seedlings [J]. Chinese Journal OF Rice Science, 2024, 38(4): 409-421. |
| [2] | XU Huan, ZHOU Tao, SUN Yue, WANG Mumei, YANG Yachun, MA Hui, LI Hao, XU Dawei, ZHOU Hai, YANG Jianbo, NI Jinlong. Characterization and Gene Mapping of a Glume Lesion Mimic Mutant glmm1 in Rice [J]. Chinese Journal OF Rice Science, 2023, 37(5): 497-506. |
| [3] | TANG Jie, LONG Tuan, WU Chunyu, LI Xinpeng, ZENG Xiang, WU Yongzhong, HUANG Peijin. Identification and Gene Mapping of a New Photo-thermo-sensitive Male Sterile Mutant tms3650 in Rice [J]. Chinese Journal OF Rice Science, 2023, 37(1): 45-54. |
| [4] | LIANG Cheng, XIANG Xunchao, ZHANG Ouling, YOU Hui, XU Liang, CHEN Yongjun. Analyses on Agronomic Traits and Genetic Characteristics of Two New Plant-architecture Lines in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(2): 171-180. |
| [5] | YANG Jinyu, BAI Chen, DING Xiaohui, SHEN Hongfang, WANG Lei, YING Jiezheng, E Zhiguo. Genetic Analysis and Gene Mapping of a Male Sterile Mutant ms7 in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(1): 27-34. |
| [6] | Yujun ZHU, Ziwei ZUO, Zhenhua ZHANG, Yeyang FAN. A New Approach for Fine-mapping and Map-based Cloning of Minor-Effect QTL in Rice [J]. Chinese Journal OF Rice Science, 2021, 35(4): 407-414. |
| [7] | Wei LIU, Zhanhua LU, Dongbai LU, Xiaofei WANG, Shiguang WANG, Jia XUE, Xiuying HE. Location and Candidate Gene Analysis of Rice Clustered Spikelets Gene OsCL6 [J]. Chinese Journal OF Rice Science, 2021, 35(3): 238-248. |
| [8] | Xuemei DENG, Peng HU, Yueying WANG, Yi WEN, Yiqing TAN, Hao WU, Kaixiong WU, Junge WANG, Linlin HOU, Lixin ZHU, Li ZHU, Guang CHEN, Dali ZENG, Guangheng ZHANG, Longbiao GUO, Zhenyu GAO, Deyong REN, Qian QIAN, Jiang HU. Identification and Fine Mapping of a Grain Width Mutant gw4 in Rice [J]. Chinese Journal OF Rice Science, 2021, 35(3): 259-268. |
| [9] | Shufang LIU, Liying DONG, Xundong LI, Wumin ZHOU, Qinzhong YANG. Different Reactions of Rice Monogenic Line IRBL9-W Harboring Pi9 Gene to Magnaporthe oryzae Containing AvrPi9 During Seedling and Adult-plant Stages [J]. Chinese Journal OF Rice Science, 2021, 35(3): 303-310. |
| [10] | Yali ZHENG, Linchuang YU, Xiaoxiao AN, Xinle CHENG, Lijun REN, Zilong SU, Xiaoya ZHENG, Tao LAN. Identification of a Knockout Mutant of OsWOX3B Gene in Rice (Oryza sativa L.) [J]. Chinese Journal OF Rice Science, 2021, 35(2): 112-120. |
| [11] | Zhonghao WANG, Yan HE, Xiaobo ZHANG, Xia XU, WUJianli, SHIYongfeng. Genetic Analysis and Gene Mapping of a Virescent and Panicle AbortionMutant vpa1in Rice [J]. Chinese Journal OF Rice Science, 2021, 35(1): 19-26. |
| [12] | Yiwei KANG, Yuyu CHEN, Yingxin ZHANG. Research Progress and Breeding Prospects of Grain Size Associated Genes in Rice [J]. Chinese Journal OF Rice Science, 2020, 34(6): 479-490. |
| [13] | Tao XIONG, Yuanyuan NIE, Fangming MAO, Jianguo LEI, Linghua MAO, Shan ZHU, Renliang HUANG, Xianhua SHEN, Song YAN. Identification and Genetic Analysis of Cross-incompatibility ina RiceDW-type Sterile Line [J]. Chinese Journal OF Rice Science, 2020, 34(6): 520-524. |
| [14] | Fudeng HUANG, Chaoyue ZHAO, Xin WU, Huanhuan HE, Fangmin CHENG, Chunshou LI, Gang PAN. Physiological Characters and Gene Mapping of a Yellow Leaf and Early-senescence Mutant osyes1 in Rice [J]. Chinese Journal OF Rice Science, 2020, 34(4): 307-315. |
| [15] | Changjian WANG, Long CHEN, Liping DAI, Xueli LU, Jinli HE, Long YANG, Jiang HU, Li ZHU, Guojun DONG, Guangheng ZHANG, Zhenyu GAO, Deyong REN, Guang CHEN, Lan SHEN, Qiang ZHANG, Longbiao GUO, Qian QIAN, Dali ZENG. Identification and Fine Mapping of Defective Pistil and Stamens 2 in Rice [J]. Chinese Journal OF Rice Science, 2020, 34(2): 115-124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||