
Chinese Journal OF Rice Science ›› 2021, Vol. 35 ›› Issue (2): 112-120.DOI: 10.16819/j.1001-7216.2021.0602
• Research Papers • Previous Articles Next Articles
Yali ZHENG1, Linchuang YU1, Xiaoxiao AN1, Xinle CHENG1, Lijun REN1, Zilong SU1, Xiaoya ZHENG1, Tao LAN1,2,3,*( )
)
Received:2020-06-02
Revised:2020-09-04
Online:2021-03-10
Published:2021-03-10
Contact:
Tao LAN
郑亚莉1, 余林闯1, 安肖肖1, 程心乐1, 任丽君1, 苏子龙1, 郑小雅1, 兰涛1,2,3,*( )
)
通讯作者:
兰涛
基金资助:Yali ZHENG, Linchuang YU, Xiaoxiao AN, Xinle CHENG, Lijun REN, Zilong SU, Xiaoya ZHENG, Tao LAN. Identification of a Knockout Mutant of OsWOX3B Gene in Rice (Oryza sativa L.)[J]. Chinese Journal OF Rice Science, 2021, 35(2): 112-120.
郑亚莉, 余林闯, 安肖肖, 程心乐, 任丽君, 苏子龙, 郑小雅, 兰涛. 一份水稻OsWOX3B基因敲除突变体的鉴定[J]. 中国水稻科学, 2021, 35(2): 112-120.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2021.0602
| 再生株系 | 阳性株系 | 阳性率 | 编辑株系 | 编辑率 | 纯合株系 | 杂合株系 |
|---|---|---|---|---|---|---|
| Regenerated lines | Positive lines | Positive rate/% | Edited lines | Editing rate/% | Homozygous lines | Heterozygous lines |
| 18 | 18 | 100 | 12 | 66.7 | 2 | 10 |
Table 1 Molecular identification results of OsWOX3B knockout T0 generation lines.
| 再生株系 | 阳性株系 | 阳性率 | 编辑株系 | 编辑率 | 纯合株系 | 杂合株系 |
|---|---|---|---|---|---|---|
| Regenerated lines | Positive lines | Positive rate/% | Edited lines | Editing rate/% | Homozygous lines | Heterozygous lines |
| 18 | 18 | 100 | 12 | 66.7 | 2 | 10 |
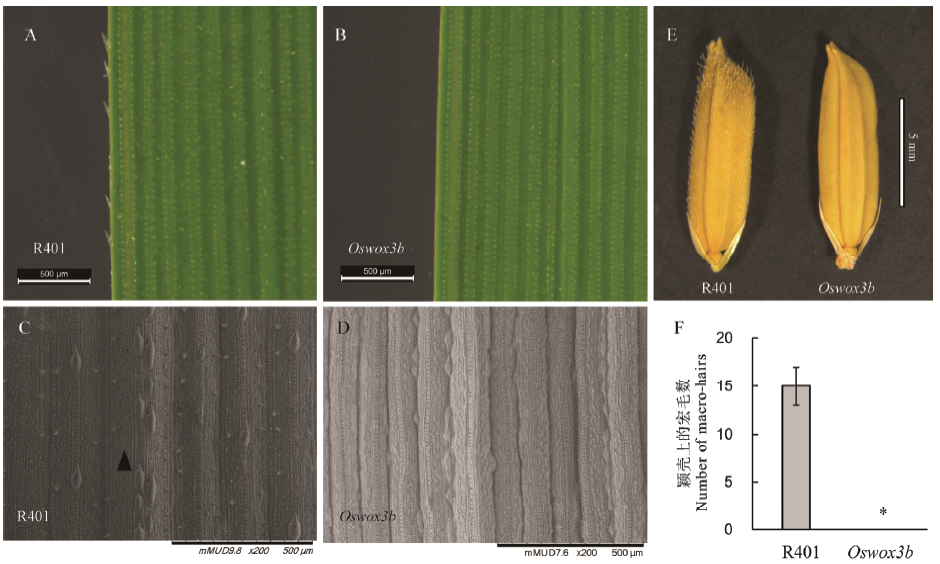
Fig. 2. Analysis of the number of epidermal hairs on the leaf and seed glume of Oswox3b mutant. A, B and E show the microscopic observation results of leaf and mature seed of wild-type R401 and homozygous mutants in tillering stage; C and D show the tillering stage leaf observation results in wild-type R401 and homozygous mutants by SEM (the arrow: macro-hair). F shows the macro-hair number in the middle adaxial surface of wild type R401 and oswox3b. The data points on the chart are mean ± standard deviation (n=3). *shows the difference is significant at P < 0.05 level.
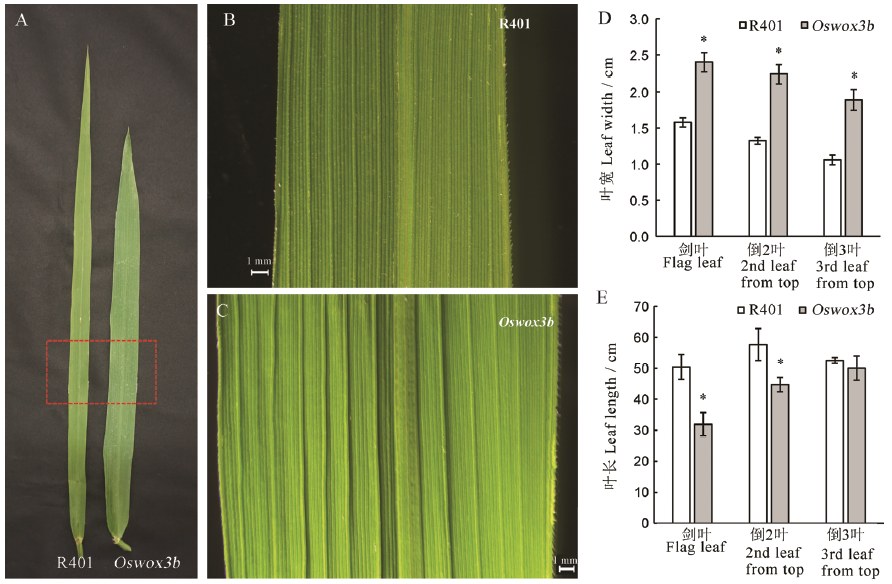
Fig. 3. Leaf length and leaf width of mutant Oswox3b. A, Wild type R401 and mutant Oswox3b at heading stage; B, C, The red area in A under a body microscope; D, Leaf width of wild type R401 and mutant Oswox3b at heading stage; E, Leaf length of wild type R401 and mutant Oswox3b at heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference is significant at P < 0.05 level.
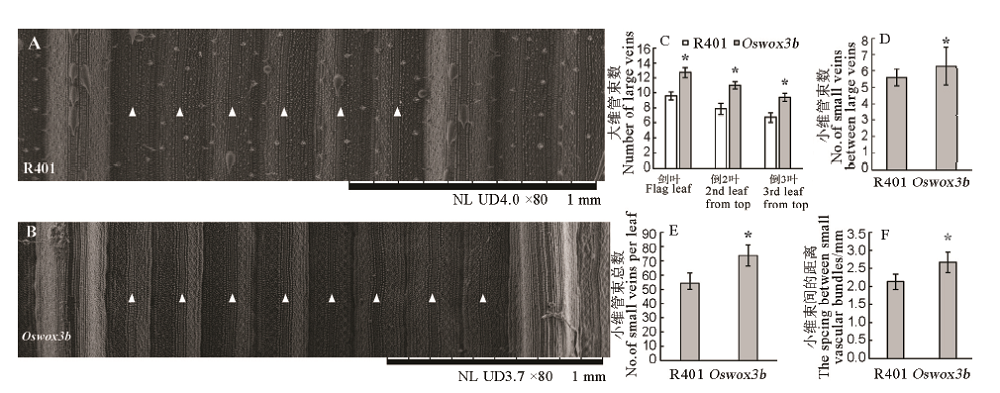
Fig. 4. Analysis of vascular bundles in leaves of mutant Oswox3b. A and B, Small vascular bundle between the wild type (A) and the mutant (B) leaf at heading stage by SEM. White triangle represents small vascular bundle; C, Large vascular bundle quantity at heading stage; D, Small vascular bundle quantity between the large vascular bundles of the flag leaf at heading stage; E, Total small vascular bundle quantity of the flag leaf at heading stage; F, Spacing between small vascular bundles of the flag leaf at heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference was significant at P < 0.05 level.
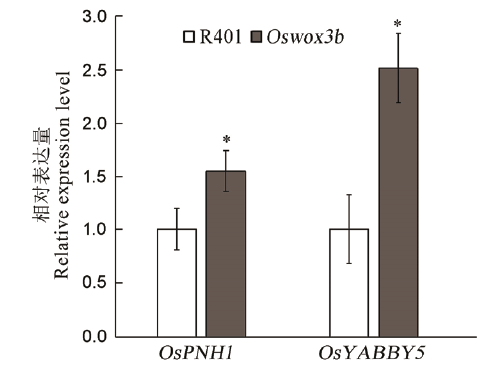
Fig. 5. The mutant Oswox3b affected the expression of genes related to vascular bundle development. The data points on the column chart are mean value ± standard deviation (n=3); *shows the difference was significant at P < 0.05 level.
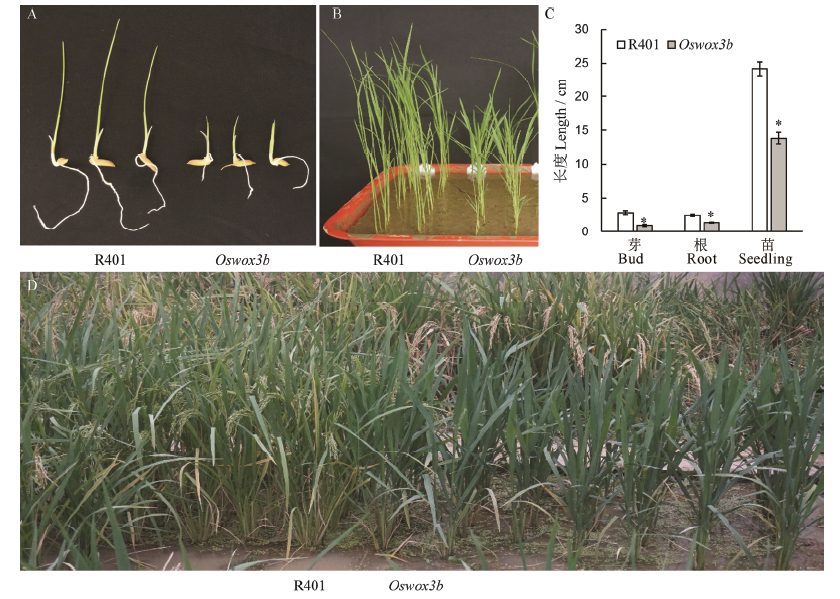
Fig. 6. Comparison of phenotypes between wild type R401 and mutant Oswox3b at various growth stages. A, B, Phenotypes of wild type and mutant in bud stage and seedling stage, respectively; C, Bud length, root length and plant height in A and B; D, Field phenotype of wild type and mutant plants in heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference was significant at P < 0.05 level.
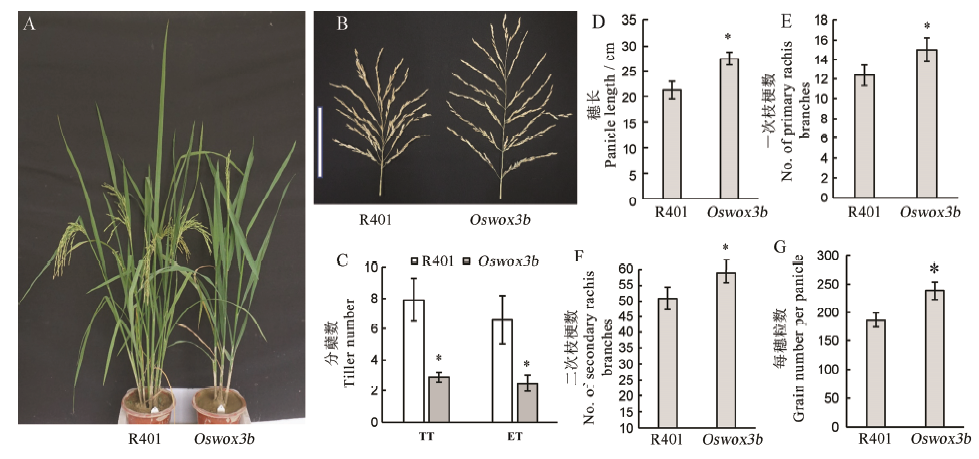
Fig. 7. Tiller, panicle and 1000-grain weight of mutant Oswox3b. A, Plant phenotype of wild type and mutant at heading stage; B, Comparison of panicle shape between wild type R401 and mutant Oswox3b (scale: 15 cm); C, Statistical analysis of tiller number and plant height of wild type R401 and mutant Oswox3b; D–G, Statistical analysis of panicle length, primary rachis branch number, secondary rachis branch number and total grain number of wild type and mutant, respectively. The data points on the column chart are mean value ± standard deviation (n=10); *Means the difference was significant at P < 0.05 level.
| [1] | 矫永庆. 水稻理想株型基因Ideal Plant Architecture 1(IPA1)的克隆与功能研究[D]. 北京: 中国科学院, 2010. |
| Jiao Y Q.Cloning and functional study of ideal plant architecture 1(IPA1)[D]. Beijing: Chinese Academy of Sciences, 2010. (in Chinese with English abstract). | |
| [2] | 于燕杰, 张大兵, 袁政. WOX蛋白家族调控干细胞发育分子机制的研究进展[J]. 植物学报, 2016, 51(4): 565-574. |
| Yu Y J, Zhang D B, Yuan Z.Progress in the molecular mechanism of WOX protein family regulating stem cell development[J]. Chinese Bulletin of Botany, 2016, 51(4): 565-574. (in Chinese with English abstract) | |
| [3] | 高丽, 孙祎敏, 邵铁梅, 孔卫娜, 崔润丽, 卢楠, 仵陶. 植物WUSCHEL-related homeobox (WOX) 家族研究进展[J]. 生物技术通报, 2015, 31(5): 7-12. |
| Gao L, Sun W M, Shao T M, Kong W N, Cui R L, Lu N, Wu T.Research progress of WUSCHEL related homeobox (WOX) family of plants[J]. Biotechnology Bulletin, 2015, 31(5): 7-12. (in Chinese with English abstract) | |
| [4] | Zhao Y, Hu Y, Dai M, Huang L, Zhou D X.The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice[J]. Plant Cell, 2009, 21(3): 736-748. |
| [5] | Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou D X.The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling[J]. Plant Cell, 2015, 27(9): 2469-2483. |
| [6] | Chen G, Feng H, Hu Q, Qu H, Chen A, Yu L, Xu G.Improving rice tolerance to potassium deficiency by enhancing OsHAK16p: WOX11-controlled root development[J]. Plant Biotechnology Journal, 2015, 13(6): 833-848. |
| [7] | Kamiya N, Nagasaki H, Morikami A, Sato Y, Matsuoka M.Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem[J]. Plant Journal, 2003, 35(4): 429-441. |
| [8] | Chu H W, Liang W Q, Li J, Hong F, Wu Y F, Wang L K, Wang J, Wu P, Liu C M, Zhang Q F, Xu J, Zhang D B.A CLE-WOX signaling module regulates root meristem maintenance and vascular tissue development in rice[J]. Journal of Experimental Botany, 2013, 64: 5359-5369. |
| [9] | Wang W F, Li G, Zhao J, Chu H W, Lin W H, Zhang D B, Wang Z Y, Liang W Q.DWARF TILLER1, a WUSCHEL-related homeobox transcription factor, is required for tiller growth in rice[J/OL]. PLoS Genetics, 2014, 10: e1004154. |
| [10] | Lu Z F, Shao G N, Xiong J S, Jiao Y Q, Wang J, Liu G F, Meng X B, Liang Y, Xiong G S, Wang Y H, Li J Y.MONOCULM 3, an ortholog of WUSCHEL in rice, is required for tiller bud formation[J]. Journal of Genetics and Genomics, 2015, 42(2): 71-78. |
| [11] | Yasui Y, Ohmori Y, Takebayashi Y, Sakakibara H, Hirano H Y.WUSCHEL-RELATED HOMEOBOX4 acts as a key regulator in early leaf development in rice[J/OL].PLoS Genetics, 2018, 14: e1007365. |
| [12] | Cho S H, Kang K, Lee S H, Lee I J, Paek N C.OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa)[J]. Journal of Experimental Botany, 2016, 67(6): 1677-1687. |
| [13] | Cho S H, Yoo S C, Zhang H, Pandeya D, Koh H J, Hwang J Y, Kim G T, Paek N C.The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development[J]. New Phytologist, 2013, 198(4): 1071-1084. |
| [14] | Dai M, Hu Y, Zhao Y, Liu H, Zhou D X.A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development[J]. Plant Physiology, 2007, 144(1): 380-390. |
| [15] | Obara M, Ikeda K, Itoh J I, Nagato Y.Characterization of leaf lateral symmetry 1 mutant[J]. Breeding Science, 2004, 54(2): 157-163. |
| [16] | Honda E, Yew C L, Yoshikawa T, Sato Y, Hibara K I, Itoh J I.LEAF LATERAL SYMMETRY1, a member of the WUSCHEL-RELATED HOMEOBOX3 gene family, regulates lateral organ development differentially from other paralogs, NARROW LEAF2 and NARROW LEAF3 in rice[J]. Plant Cell Physiology, 2018, 59(2): 376-391. |
| [17] | Angeles-Shim R B, Asano K, Takashi T, Shim J, Kuroha T, Ayano M, Ashikari M. A WUSCHEL-related homeobox 3B gene, depilous (dep), confers glabrousness of rice leaves and glumes[J]. Rice, 2012, 5(1): 28. |
| [18] | Zhang H L, Wu K, Wang Y F, Peng Y, Hu F Y, Wen L, Han B, Qian Q, Teng S.A WUSCHEL-like homeobox gene, OsWOX3B responses to NUDA/GL-1 locus in rice[J]. Rice, 2012, 5(1): 30. |
| [19] | Li J, Yuan Y, Lu Z, Yang L, Gao R, Lu J, Li J, Xiong G.Glabrous rice 1, encoding a homeodomain protein, regulates trichome development in rice[J]. Rice, 2012, 5(1): 32. |
| [20] | Sun W Q, Gao D W, Xiong Y, Tang X X, Xiao X F, Wang C G, Yu S B.Hairy Leaf 6, an AP2/ERF transcription factor, interacts with OsWOX3B and regulates trichome formation in rice[J]. Molecular Plant, 2017, 10(11): 1417-1433. |
| [21] | Nishimura A, Ito M, Kamiya N, Sato Y, Matsuoka M.OsPNH1 regulates leaf development and maintenance of the shoot apical meristem in rice[J]. Plant Journal, 2002, 30(2): 189-201. |
| [22] | Tanaka W, Toriba T, Ohmori Y, Yoshida A, Kawai A, Mayama-Tsuchida T, Ichikawa H, Mitsuda N, Ohme-Takagi M, Hirano H Y.The YABBY gene TONGARI-BOUSHI1 is involved in lateral organ development and maintenance of meristem organization in the rice spikelet[J]. Plant Cell, 2012, 24(1): 80-95. |
| [23] | Ishiwata A, Ozawa M, Nagasaki H, Kato M, Noda Y, Yamaguchi T, Nosaka M, Shimizu-Sato S, Nagasaki A, Maekawa M, Hirano H Y, Sato Y.Two WUSCHEL-related homeobox genes, narrow leaf 2 and narrow leaf 3, control leaf width in rice[J]. Plant and Cell Physiology, 2013, 54(5): 779-792. |
| [24] | Chen M, Luo J, Shao G, Wei X, Tang S, Sheng Z, Song J, Hu P.Fine mapping of a major QTL for flag leaf width in rice, qFLW4, which might be caused by alternative splicing of NAL1[J]. Plant Cell Reports, 2012, 31(5): 863-872. |
| [25] | Qi J, Qian Q, Bu Q, Li S, Chen Q, Sun J, Liang W, Zhou Y, Chu C, Li X, Ren F, Palme K, Zhao B, Chen J, Chen M, Li C.Mutation of the rice narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport[J]. Plant Physiology, 2008, 147(4): 1947-1959. |
| [26] | Zhang X, Zong J, Liu J L, Yin J Y, Zhang D B.Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar[J]. Journal of Integrative Plant Biology, 2010, 52(11): 1016-1026. |
| [27] | Yoo S C, Cho S H, Paek N C. Rice WUSCHEL-related homeobox 3A (OsWOX3A) modulates auxin-transport gene expression in lateral root and root hair development[J/OL]. Plant Signaling and Behavior, 2013, 8(10): 10, e25929. |
| [1] | HOU Xiaoqin, WANG Ying, YU Bei, FU Weimeng, FENG Baohua, SHEN Yichao, XIE Hangjun, WANG Huanran, XU Yongqiang, WU Zhihai, WANG Jianjun, TAO Longxing, FU Guanfu. Mechanisms Behind the Role of Potassium Fulvic Acid in Enhancing Salt Tolerance in Rice Seedlings [J]. Chinese Journal OF Rice Science, 2024, 38(4): 409-421. |
| [2] | LIANG Cheng, XIANG Xunchao, ZHANG Ouling, YOU Hui, XU Liang, CHEN Yongjun. Analyses on Agronomic Traits and Genetic Characteristics of Two New Plant-architecture Lines in Rice [J]. Chinese Journal OF Rice Science, 2022, 36(2): 171-180. |
| [3] | Yujun ZHU, Ziwei ZUO, Zhenhua ZHANG, Yeyang FAN. A New Approach for Fine-mapping and Map-based Cloning of Minor-Effect QTL in Rice [J]. Chinese Journal OF Rice Science, 2021, 35(4): 407-414. |
| [4] | Yiwei KANG, Yuyu CHEN, Yingxin ZHANG. Research Progress and Breeding Prospects of Grain Size Associated Genes in Rice [J]. Chinese Journal OF Rice Science, 2020, 34(6): 479-490. |
| [5] | Yanhua CHEN, Yaliang WANG, Defeng ZHU, Qinghua SHI, Huizhe CHEN, Jing XIANG, Yikai ZHANG, Yuping ZHANG. Mechanism of Exogenous Brassinolide in Alleviating High Temperature Injury at Panicle Initiation Stage in Rice [J]. Chinese Journal OF Rice Science, 2019, 33(5): 457-466. |
| [6] | Jingfang LI, Yunlu TIAN, Xi LIU, Shijia LIU, Liangming CHEN, Ling JIANG, Wenwei ZHANG, Dayong XU, Yihua WANG, Jianmin WAN. The Guanylate Kinase OsGK1 is Essential for Seed Development in Rice [J]. Chinese Journal OF Rice Science, 2018, 32(5): 415-426. |
| [7] | Peng-yi PAN, Jian-ping ZHU, Yun-long WANG, Yuan-yuan HAO, Yue CAI, Wen-wei ZHANG, Ling JIANG, Yi-hua WANG, Jian-min WAN. Phenotyping and Gene Cloning of a Floury Endosperm Mutant ws in Rice [J]. Chinese Journal OF Rice Science, 2016, 30(5): 447-457. |
| [8] | Min XI, Zhao-miao LIN, Yan-ling ZHAO, Xin-cheng ZHANG, Xiao-yu YANG, Zheng-hui LIU, Gang-hua LI, Shao-hua WANG, Yan-feng DING. Effects of Nitrogen Fertilizer Application on the Formation of White-belly and White-core as Well as Biochemical Composition of japonica Rice Grains [J]. Chinese Journal OF Rice Science, 2016, 30(2): 193-199. |
| [9] | XUQian-kun, De-yong REN, Zi-zhuang LI, Da-li ZENG, Long-biao GUO, Qian QIAN. Research Progresses in Rice Spikelet Glume Development [J]. Chinese Journal OF Rice Science, 2016, 30(1): 99-104. |
| [10] | Peng WANG, Yue CAI, Wei-wei CHEN, Jing MA, Xin-gang CHEN, Xiao-jie TANG, Xiao-man YOU, Fei KONG, Jie ZHANG, Hai-gang YAN, Guo-xiang WANG, Ling JIANG, Wen-wei ZHANG, Jian-min WAN. Phenotyping and Gene Cloning of a Small-grain Dwarf Mutant sgd1(t) in Rice [J]. Chinese Journal OF Rice Science, 2016, 30(1): 1-9. |
| [11] | Zuo-zhen DONG, Liang-huan WU, Jie CHAI, Yuan-li CHEN, Yue-zhong ZHU. Effects of Different Nitrogen, Phosphorus and Potassium Treatments on Rice Yield, Quality, Nutrient Absorption-Utilization and Economic Benefit of Zhongzheyou 1 in Central Zhejiang Province,China [J]. Chinese Journal OF Rice Science, 2015, 29(4): 399-407. |
| [12] | Da SU, Fu-biao WANG, Bing-ting LEI, Jue WANG, Gang PAN, Fang-min CHENG. The Response of Phytic Acid and Its Expression Profiles in Rice (Oryza sativa L.) Grain as Induced by Phosphorus Supply [J]. Chinese Journal OF Rice Science, 2015, 29(2): 159-166. |
| [13] | GONG Jinlong, XING Zhipeng, HU Yajie, ZHANG Hongcheng*, DAI Qigen, HUO Zhongyang, XU Ke, WEI Haiyan, GAO Hui. Difference in Growth Duration and Utilization of Temperature and Solar Radiation Between indica and japonica Super Rice in the Lower Yangtze and Huaihe River Valley [J]. Chinese Journal of Rice Science, 2014, 28(3): 267-276. |
| [14] | LONG Xiaolin, XIANG Xunchao*, XU Yanfang, SU Wenli, KANG Cuifang. Absorption, Transfer and Distribution of Cd in indica and japonica Rice under Cd Stress [J]. Chinese Journal of Rice Science, 2014, 28(2): 177-184. |
| [15] | DONG Guichun, CHEN Chen, WANG Yi, ZHONG Jun, YUAN Qiumei, YANG Bing, YU Xiaofeng, LI Jinqian, TIAN Hao, ZHANG Yan, JIANG Yaming, MENG Lingxiang, WANG Yulong*. Relation Between Root Traits and Growth Duration in japonica Rice Cultivars [J]. Chinese Journal of Rice Science, 2013, 27(4): 398-404. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||