
中国水稻科学 ›› 2023, Vol. 37 ›› Issue (4): 368-378.DOI: 10.16819/j.1001-7216.2023.221112
汪胜勇1, 陈宇航1, 陈会丽1, 黄钰杰1, 张啸天1, 丁双成2( ), 王宏伟1(
), 王宏伟1( )
)
收稿日期:2022-11-24
修回日期:2023-02-07
出版日期:2023-07-10
发布日期:2023-07-17
通讯作者:
*email: shchding@yangtzeu.edu.cn;wanghw@yangtzeu.edu.cn
基金资助:
WANG Shengyong1, CHEN Yuhang1, CHEN Huili1, HUANG Yujie1, ZHANG Xiaotian1, DING Shuangcheng2( ), WANG Hongwei1(
), WANG Hongwei1( )
)
Received:2022-11-24
Revised:2023-02-07
Online:2023-07-10
Published:2023-07-17
Contact:
*email: shchding@yangtzeu.edu.cn;wanghw@yangtzeu.edu.cn
摘要:
【目的】 探究水稻减数分裂期高温如何影响苯丙烷类代谢,并分析其与水稻耐热性的关系。【方法】 以N22、广陆矮15、SDWG005、全两优681、Y两优900、Y两优1号、两优培九和绵恢101(MH101)等8种耐热和不耐热水稻品种为试验材料,设置常温和高温处理,分析减数分裂期高温胁迫对水稻的花粉活力与苯丙烷类代谢关键酶活性、木质素、总黄酮及总酚等主要代谢产物含量之间的相关性;并进一步选择极端耐高温的SDWG005和极端不耐高温的MH101为材料分析苯丙烷类代谢、碳水化合物代谢和抗氧化系统对水稻耐热性的影响。【结果】 1) 与对照相比,高温显著降低水稻花粉活力和颖花受精率,不同的水稻品种受高温影响后,花粉活力和颖花受精率的降幅不同。2) 高温显著增加颖花中肉桂酸-4-羟化酶和4-香豆酸辅酶A连接酶活性以及木质素、总黄酮和总酚的含量,且耐热品种增幅高于敏感品种。3) 高温下花粉活力与肉桂酸-4-羟化酶活性、木质素含量显著相关,颖花受精率与木质素含量以及木质素含量与类黄酮含量极显著相关。4) 与MH101相比,SDWG005小穗颖壳中木质素受高温显著诱导积累,且高温下能够维持较高细胞壁过氧化物酶活性。5) 与MH101相比,SDWG005颖花在高温下能够维持较高过氧化物酶、超氧化物歧化酶和抗坏血酸氧化酶活性,进而减少颖花中过氧化氢和丙二醛的积累。高温下SDWG005颖花中淀粉含量更高,酸性转化酶、蔗糖合酶及ATPase基因的表达量显著增加。【结论】 减数分裂期高温促进颖花中苯丙烷类代谢关键酶活性的上升和代谢产物含量的增加,耐热品种高温下能够积累较多的木质素和类黄酮,具有较高抗氧化酶活性,同时蔗糖代谢和能量产生效率较高,从而具有较强的耐热性。
汪胜勇, 陈宇航, 陈会丽, 黄钰杰, 张啸天, 丁双成, 王宏伟. 水稻减数分裂期高温对苯丙烷类代谢及下游分支代谢途径的影响[J]. 中国水稻科学, 2023, 37(4): 368-378.
WANG Shengyong, CHEN Yuhang, CHEN Huili, HUANG Yujie, ZHANG Xiaotian, DING Shuangcheng, WANG Hongwei. Effects of High Temperature on Phenylpropane Metabolism and Downstream Branch Metabolic Pathways in Rice Meiosis[J]. Chinese Journal OF Rice Science, 2023, 37(4): 368-378.
| 基因位点号(基因名) Locus ID(gene name) | 正向引物序列 Forward(5'-3') | 反向引物 Reverse(5'-3') |
|---|---|---|
| LOC_Os09g08072(INV1) | AAGAGCAGGGGTGTACAAG | AAGCTGTGAGTCTGTGGCTC |
| LOC_Os06g09450(SUS2) | GTGTGCTTGACACCATCCAC | CATGCGGAGACAGGATAACA |
| LOC_Os05g45420(SnRK1A) | ACAACCAGTGGCTACCTTGG | CGATGATCAGTGGCTGAGTT |
| LOC_Os07g09610(SnRK1B) | ATATCAGGCGCCGAATACTG | TGTGCCTGAAGAACTTGCTG |
| LOC_Os05g14550(TOR) | GCTGAACGCTGCAATGACTA | ACCGAACAAGTACTGGAGCA |
| NC_001320.1(ATPase) | TCGGTGGAGCTACTCTTGGA | CGGGCGCGGATCTATGAATA |
| Tubulin | TACCGTGCCCTTACTGTTCC | CGGTGGAATGTCACAGACAC |
表1 基因表达分析使用的引物
Table 1. Primers used in gene expression analysis.
| 基因位点号(基因名) Locus ID(gene name) | 正向引物序列 Forward(5'-3') | 反向引物 Reverse(5'-3') |
|---|---|---|
| LOC_Os09g08072(INV1) | AAGAGCAGGGGTGTACAAG | AAGCTGTGAGTCTGTGGCTC |
| LOC_Os06g09450(SUS2) | GTGTGCTTGACACCATCCAC | CATGCGGAGACAGGATAACA |
| LOC_Os05g45420(SnRK1A) | ACAACCAGTGGCTACCTTGG | CGATGATCAGTGGCTGAGTT |
| LOC_Os07g09610(SnRK1B) | ATATCAGGCGCCGAATACTG | TGTGCCTGAAGAACTTGCTG |
| LOC_Os05g14550(TOR) | GCTGAACGCTGCAATGACTA | ACCGAACAAGTACTGGAGCA |
| NC_001320.1(ATPase) | TCGGTGGAGCTACTCTTGGA | CGGGCGCGGATCTATGAATA |
| Tubulin | TACCGTGCCCTTACTGTTCC | CGGTGGAATGTCACAGACAC |
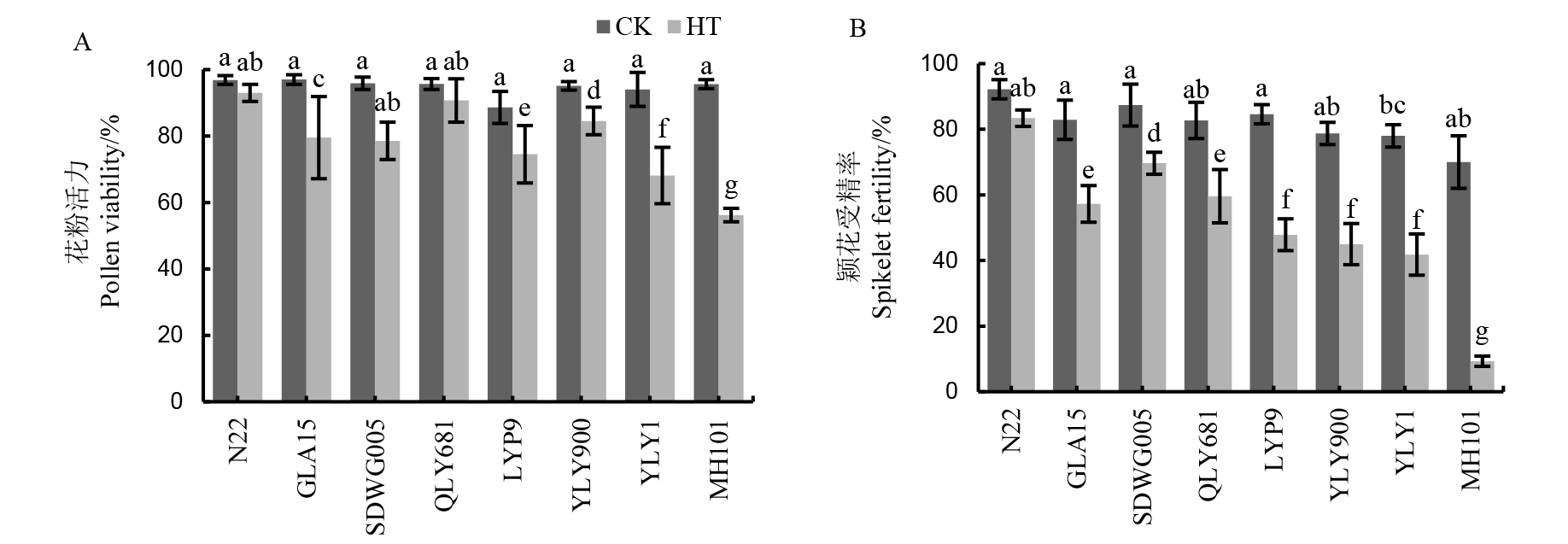
图2 不同水稻品种减数分裂期高温处理下的花粉活力(A)和颖花受精率(B) GLA15-广陆矮15;QLY681-全两优681;LYP9-两优培九;YLY900-Y两优900;YLY1-Y两优1号;MH101-绵恢101。平均值±标准差,n = 3,柱状图上不同小写字母表示不同品种及处理间差异达5%显著水平。下同。
Fig. 2. Pollen vitality (A) and spikelet fertility (B) of different rice cultivars under HT during meiosis stage. GLA15, Guanglu’ai 15; QLY681, Quanliangyou 681; LYP9, Liangyoupeijiu; YLY900, Y Liangyou 900; YLY1, Y Liangyou 1; MH101, Mianhui 101. Mean ± standard deviation, n = 3; different lowercase letters on the bars indicate significant difference among varieties and treatments at 5% level. The same below.
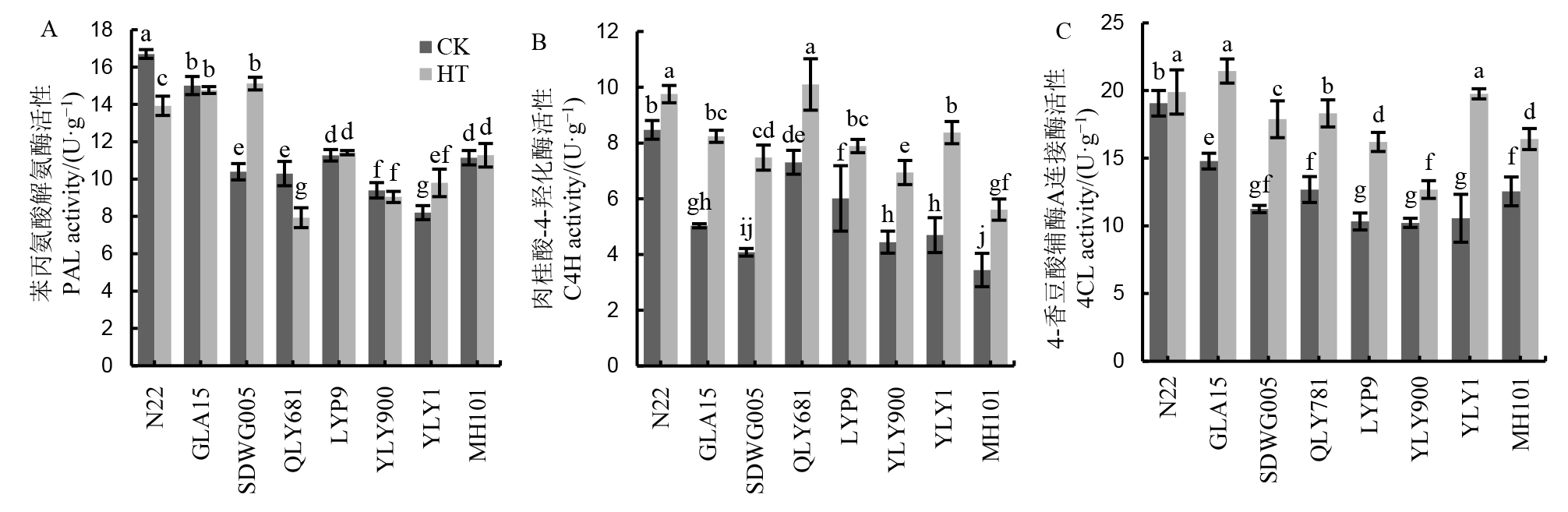
图3 不同水稻品种减数分裂期高温处理下苯丙烷类代谢途径关键酶活性比较
Fig. 3. Comparison of key enzyme activities involved in phenylpropanoid pathway in different rice cultivars under HT during meiosis stage.
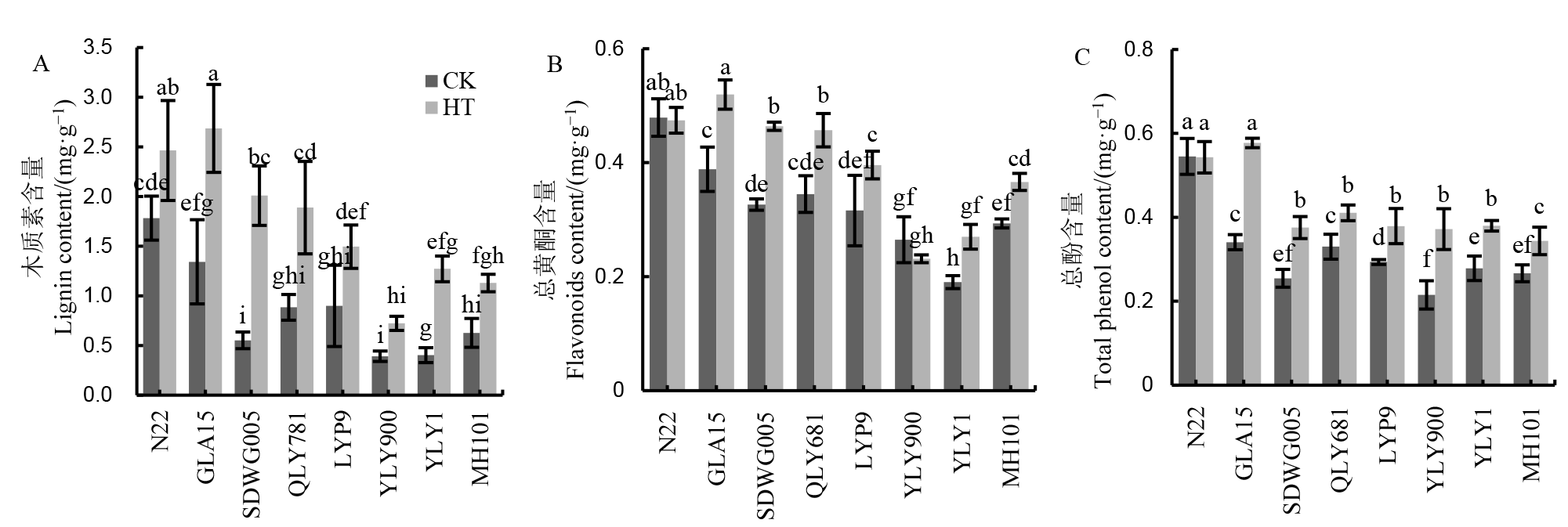
图4 不同水稻品种减数分裂期高温处理下颖花木质素、总黄酮和总酚含量的比较
Fig. 4. Comparison of lignin, flavonoids and total phenol contents in different rice cultivars under HT during meiosis stage.
| 相关系数 Correlation coefficient | 花粉活力 Pollen vitality | 颖花受精率 Spikelet fertility | 苯丙氨酸 解氨酶活性 Phenylalanine ammonia lyase activity | 肉桂酸-4- 羟化酶活性 Cinnamic acid- 4-hydroxylase activity | 4-香豆酸辅酶 A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 总酚 Total phenols | 类黄酮 Flavonoids | 木质素 Lignin | |
|---|---|---|---|---|---|---|---|---|---|
| CK | 花粉活力Pollen viability | 1.000 | |||||||
| 颖花受精率Spikelet fertility | 0.615 | 1.000 | |||||||
| 苯丙氨酸解氨酶活性 Phenylalanine ammonialyase activity | 0.193 | 0.662 | 1.000 | ||||||
| 肉桂酸-4-羟化酶活性 Cinnamic acid-4-hydroxylase activity | 0.499 | 0.505 | 0.545 | 1.000 | |||||
| 4-香豆酸辅酶A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 0.406 | 0.634 | 0.910** | 0.648 | 1.000 | ||||
| 总酚Total phenols | 0.446 | 0.596 | 0.836** | 0.840** | 0.930** | 1.000 | |||
| 总黄酮Flavonoids | 0.253 | 0.510 | 0.872** | 0.412 | 0.832** | 0.709* | 1.000 | ||
| 木质素Lignin | 0.334 | 0.576 | 0.918** | 0.632 | 0.904** | 0.864** | 0.960** | 1.000 | |
| HT | 花粉活力Pollen vitality | 1.000 | |||||||
| 颖花受精率Spikelet fertility | 0.905** | 1.000 | |||||||
| 苯丙氨酸解氨酶活性 Phenylalanine ammonialyase activity | 0.188 | 0.499 | 1.000 | ||||||
| 肉桂酸-4-羟化酶活性 Cinnamic acid-4-hydroxylase activity | 0.838** | 0.694 | 0.068 | 1.000 | |||||
| 4-香豆酸辅酶A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 0.416 | 0.403 | 0.485 | 0.546 | 1.000 | ||||
| 总酚Total phenols | 0.561 | 0.629 | 0.539 | 0.539 | 0.693 | 1.000 | |||
| 总黄酮Flavonoids | 0.562 | 0.588 | 0.659 | 0.406 | 0.733* | 0.662 | 1.000 | ||
| 木质素Lignin | 0.775* | 0.887** | 0.649 | 0.598 | 0.590 | 0.668 | 0.860** | 1.000 | |
表2 CK和HT处理下苯丙烷类代谢及下游分支代谢途径相关指标相关性分析
Table 2. Correlation analysis of various indexes involved in phenylpropane metabolism and downstream branch metabolic pathways under CK and HT.
| 相关系数 Correlation coefficient | 花粉活力 Pollen vitality | 颖花受精率 Spikelet fertility | 苯丙氨酸 解氨酶活性 Phenylalanine ammonia lyase activity | 肉桂酸-4- 羟化酶活性 Cinnamic acid- 4-hydroxylase activity | 4-香豆酸辅酶 A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 总酚 Total phenols | 类黄酮 Flavonoids | 木质素 Lignin | |
|---|---|---|---|---|---|---|---|---|---|
| CK | 花粉活力Pollen viability | 1.000 | |||||||
| 颖花受精率Spikelet fertility | 0.615 | 1.000 | |||||||
| 苯丙氨酸解氨酶活性 Phenylalanine ammonialyase activity | 0.193 | 0.662 | 1.000 | ||||||
| 肉桂酸-4-羟化酶活性 Cinnamic acid-4-hydroxylase activity | 0.499 | 0.505 | 0.545 | 1.000 | |||||
| 4-香豆酸辅酶A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 0.406 | 0.634 | 0.910** | 0.648 | 1.000 | ||||
| 总酚Total phenols | 0.446 | 0.596 | 0.836** | 0.840** | 0.930** | 1.000 | |||
| 总黄酮Flavonoids | 0.253 | 0.510 | 0.872** | 0.412 | 0.832** | 0.709* | 1.000 | ||
| 木质素Lignin | 0.334 | 0.576 | 0.918** | 0.632 | 0.904** | 0.864** | 0.960** | 1.000 | |
| HT | 花粉活力Pollen vitality | 1.000 | |||||||
| 颖花受精率Spikelet fertility | 0.905** | 1.000 | |||||||
| 苯丙氨酸解氨酶活性 Phenylalanine ammonialyase activity | 0.188 | 0.499 | 1.000 | ||||||
| 肉桂酸-4-羟化酶活性 Cinnamic acid-4-hydroxylase activity | 0.838** | 0.694 | 0.068 | 1.000 | |||||
| 4-香豆酸辅酶A连接酶活性 4-Coumaric acid coenzyme A ligase activity | 0.416 | 0.403 | 0.485 | 0.546 | 1.000 | ||||
| 总酚Total phenols | 0.561 | 0.629 | 0.539 | 0.539 | 0.693 | 1.000 | |||
| 总黄酮Flavonoids | 0.562 | 0.588 | 0.659 | 0.406 | 0.733* | 0.662 | 1.000 | ||
| 木质素Lignin | 0.775* | 0.887** | 0.649 | 0.598 | 0.590 | 0.668 | 0.860** | 1.000 | |
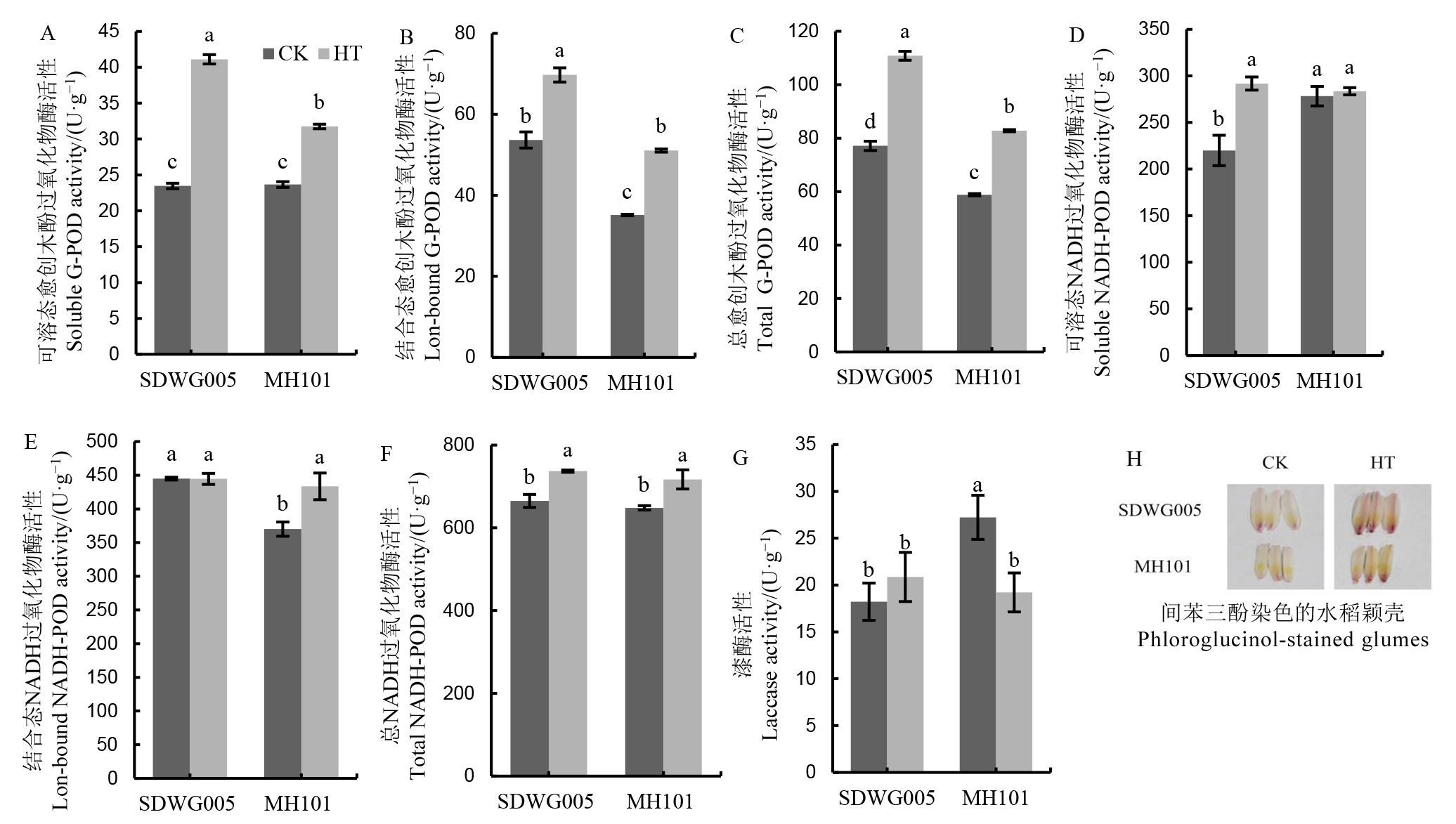
图5 水稻减数分裂期高温对颖花细胞壁过氧化物酶活性的影响 柱状图上不同小写字母表示不同品种及处理间差异达5%显著水平。
Fig. 5. Effects of HT on cell wall peoxidase activities in spikelets during meiosis stage. Different lowercase letters above the bars indicate significant difference among different varieties and treatments at 5% level.
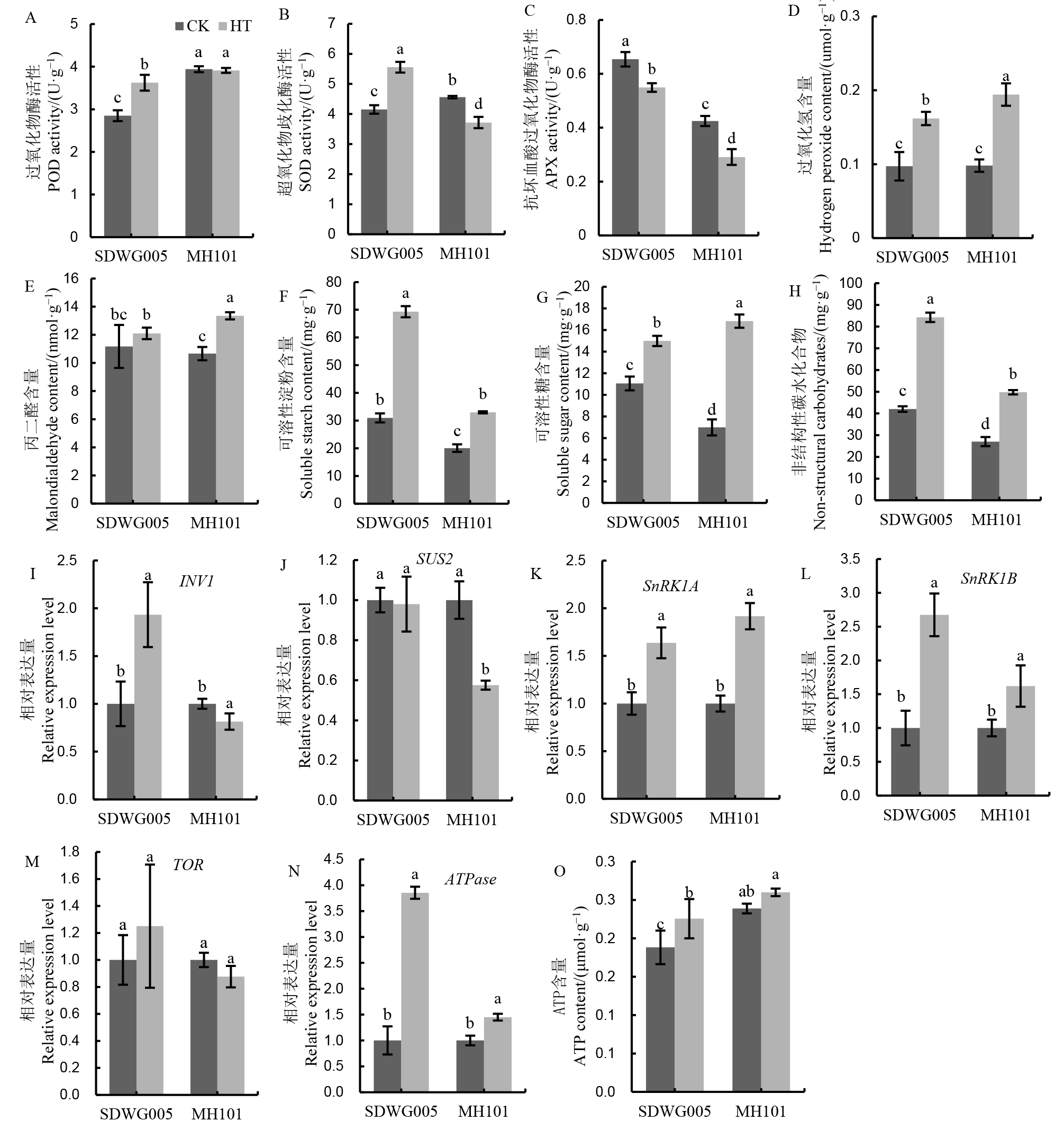
图6 减数分裂期高温对水稻颖花中抗氧化酶活性、过氧化氢、丙二醛和碳水化合物含量以及蔗糖代谢和能量水平相关基因表达的影响 柱状图上不同小写字母表示不同品种及处理间差异达5%显著水平。
Fig. 6. Effects of HT on antioxidant enzyme activities, H2O2, malondialdehyde, carbohydrate contents, and gene expression involved in sucrose metabolic and energy level in spikelets during meiosis stage. Different lowercase letters above the bars indicate significant difference among different varieties and treatments at 5% level.
| [1] | Shi P H, Tang L, Wang L H, Sun T, Liu L L, Cao W X, Zhu Y. Post-heading heat stress in rice of south China during 1981-2010[J]. PLoS One, 2015, 10(6): e0130642. |
| [2] | Shukla P R, Skeg J, Calvo Buendia E, Masson-Delmotte V, Pörtner H O, Malley J. Climate change and land:An IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems[M]. Koyoto, Japan: Intergovernmental Panel on Climate Change(IPCC), 2019. |
| [3] | 杨军, 章毅之, 贺浩华, 李迎春, 陈小荣, 边建民, 金国花, 李翔翔, 黄淑娥. 水稻高温热害的研究现状与进展[J]. 应用生态学报, 2020, 31(8): 2817-2830. |
| Yang J, Zhang Y Z, He H H, Li Y C, Chen X R, Bian J M, Jin G H, Li X X, Huang S E. Current status and research advances of high-temperature hazards in rice[J]. Chinese Journal of Applied Ecology, 2020, 31(8): 2817-2830. (in Chinese with English abstract) | |
| [4] | 田小海, 罗海伟, 吴晨阳. 中国水稻热害研究历史、进展与展望[J]. 中国农学通报, 2009, 25(22): 166-168. |
| Tian X H, Luo H W, Wu C Y. Research on heat stress of rice in China: Progress and prospect[J]. Chinese Agricultural Science Bulletin, 2009, 25(22): 166-168. (in Chinese with English abstract) | |
| [5] | Peng S B, Huang J L, Sheehy J E, Laza R C, Cassman K G. Rice yields decline with higher night temperature from global warming[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(27): 9971-9975. |
| [6] | Ray D K, Gerber J S, Macdonald G K, West P C. Climate variation explains a third of global crop yield variability[J]. Nature Communications, 2015, 6: 5989. |
| [7] | Sage T L, Bagha S, Lundsgaard-Nielsen V, Branch H A, Sultmanis S, Sage R F. The effect of high temperature stress on male and female reproduction in plants[J]. Field Crops Research, 2015, 182: 30-42. |
| [8] | Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development[J]. Plant & Cell Physiology, 2009, 50(11): 1911-22. |
| [9] | Sato S, Peet M M, Thomas J F. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures[J]. Journal of Experimental Botany, 2002, 53(371): 1187-1195. |
| [10] | Jagadish S V K, Craufurd P Q, Wheeler T R. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis[J]. Crop Science, 2008, 48(3): 1140-1146. |
| [11] | Wilson Z A, Song J, Taylor B, Yang C Y. The final split: The regulation of anther dehiscence[J]. Journal of Experimental Botany, 2011, 62(5): 1633-1649. |
| [12] | Matsui T, Omasa K, Horie T. High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.)[J]. Plant Production Science, 2000, 3(4): 430-434. |
| [13] | Matsui T, Omasa K. Rice cultivars tolerant to high temperature at flowering: Anther characteristics[J]. Annals of Botany, 2002, 89(6): 683-687. |
| [14] | Prasad P V V, Boote K J, Allen L H, Sheehy J E. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature[J]. Field Crops Research, 2006, 95(2): 398-411. |
| [15] | Jagadish S V, Muthurajan R, Oane R, Wheeler T R, Heuer S, Bennett J, Craufurd P Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.)[J]. Journal of Experimental Botany, 2010, 61(1): 143-156. |
| [16] | Kobayashi K, Matsui T, Murata Y, Yamamoto M. Percentage of dehisced thecae and length of dehiscence control pollination stability of rice cultivars[J]. Plant Production Science, 2011, 14(2): 89-95. |
| [17] | 张桂莲, 张顺堂, 肖浪涛, 唐文帮, 肖应辉, 陈立云. 抽穗开花期高温胁迫对水稻花药、花粉粒及柱头生理特性的影响[J]. 中国水稻科学, 2014, 28(2): 155-166. |
| Zhang G L, Zhang S T, Xiao L T, Tang W B, Xiao Y H, Chen L Y. Effect of high temperature stress on physiological characteristics of anther, pollen and stigma of rice during heading-flowering stage[J]. Chinese Journal of Rice Science, 2014, 28(2): 155-166. (in Chinese with English abstract) | |
| [18] | Wahid A, Gelani S, Ashraf M, Foolad M R. Heat tolerance in plants: An overview[J]. Environmental and Experimental Botany, 2007, 61(3): 199-223. |
| [19] | Locato V, de Pinto M C, de Gara L. Different involvement of the mitochondrial, plastidial and cytosolic ascorbate-glutathione redox enzymes in heat shock responses[J]. Physiologia Plantarum, 2009, 135(3): 296-306. |
| [20] | Locato V, Gadaleta C, de Gara L, de Pinto M C. Production of reactive species and modulation of antioxidant network in response to heat shock: A critical balance for cell fate[J]. Plant Cell & Environment, 2008, 31(11): 1606-1619. |
| [21] | Parish R W, Phan H A, Iacuone S, Li S F. Tapetal development and abiotic stress[J]. Functional Plant Biology, 2012, 39(7): 553-559. |
| [22] | De Storme N, Geelen D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms[J]. Plant Cell & Environment, 2014, 37(1): 1-18. |
| [23] | Bahuguna R N, Solis C A, Shi W J, Jagadish K S. Post-flowering night respiration and altered sink activity account for high night temperature-induced grain yield and quality loss in rice (Oryza sativa L.)[J]. Physiologia Plantarum, 2017, 159(1): 59-73. |
| [24] | Herrmann K M, Weaver L M. The Shikimate Pathway[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1999, 50: 473-503. |
| [25] | Schubert W J, Acerbo S N. The conversion of D-glucose into lignin in Norwegian spruce[J]. Archives of Biochemistry and Biophysics, 1959, 83(1): 178-82. |
| [26] | Rogers L A, Dubos C, Cullis I F, Surman C, Poole M, Willment J, Mansfield S D, Campbell M M. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis[J]. Journal of Experimental Botany, 2005, 56(416): 1651-63. |
| [27] | Jiao Y, Gong X, Qi K, Xie Z, Wang Y, Yuan K, Pan Q, Zhang S, Shiratake K, Khanizadeh S, Tao S. Transcriptome analysis provides new ideas for studying the regulation of glucose-induced lignin biosynthesis in pear calli[J]. BMC Plant Biology, 2022, 22(1): 310. |
| [28] | Poovaiah C R, Mazarei M, Decker S R, Turner G B, Sykes R W, Davis M F, Stewart C N, Jr. Transgenic switchgrass biomass is increased by overexpression of switchgrass sucrose synthase (PvSUS1)[J]. Biotechnology Journal, 2015, 10(4): 552-563. |
| [29] | Dong N Q, Lin H X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions[J]. Journal of Integrative Plant Biology, 2021, 63(1): 180-209. |
| [30] | Muhlemann J K, Younts T L B, Muday G K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(47): e11188-e11197. |
| [31] | Zhang D B, Wilson Z A. Stamen specification and anther development in rice[J]. Chinese Science Bulletin, 2009, 54(14): 2342-2353. |
| [32] | Fecht-Christoffers M M, Fuhrs H, Braun H P, Horst W J. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance[J]. Plant Physiology, 2006, 140(4): 1451-63. |
| [33] | Dong N Q, Sun Y, Guo T, Shi C L, Zhang Y M, Lin H X. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice[J]. Nature Communications, 2020, 11(1): 2629. |
| [34] | Liu Y Y, Ge Y H, Bi Y, Li C Y, Deng H W, Hu L G, Dong B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon fruits[J]. Scientia Horticulturae, 2014, 168: 113-119. |
| [35] | 张水明, 龚凌燕, 曹丹琴, 张永娟, 杨健. 石榴种皮总木质素含量及PgCOMT基因的克隆与表达[J]. 热带亚热带植物学报, 2015, 23(1): 65-73. |
| Zhang S M, Gong L Y, Cao D Q, Zhang Y J, Yang J. Total lignin content in pomegranate seed coat and cloning andexpression analysis of PgCOMT gene[J]. Journal of Tropical and Subtropical Botany, 2015, 23(1): 65-73. (in Chinese with English abstract) | |
| [36] | 黄晓彤, 史锐, 刘苗苗, 丛龙娇, 刘斯文, 王琪瑶. 同属不同种桑叶总黄酮的含量测定及分析[J]. 亚太传统医药, 2022, 18(8): 91-95. |
| Huang X T, Shi R, Liu M M, Cong L J, Liu S W, Wang Q Y. Optimization of extraction process and content determination of total flavonoids from different mulberry leaves[J]. Asia-Pacific Traditional Medicine, 2022, 18(8): 91-95. (in Chinese with English abstract) | |
| [37] | 王成章, 李建华, 郭玉霞, 方丽云, 高永革. 光周期对不同秋眠型苜蓿SOD、POD活性的影响[J]. 草地学报, 2007, 15(5): 407-411. (in Chinese with English abstract) |
| Wang C Z, Li J H, Guo Y X, Fang L Y, Gao Y G. Effect of photoperiod on SOD and POD activities in alfalfa varieties with different fall dormancy[J]. Acta Agrestia Sinica, 2007, 15(5): 407-411. | |
| [38] | Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specificperoxidase in spinach chloroplasts[J]. Plant and Cell Physiology, 1981, 22(5): 867-880. |
| [39] | 李光彦. 能量代谢影响水稻耐热性的作用机理[D]. 武汉: 华中农业大学, 2021. |
| Li G Y. The mechanism of energy metabolism mediating rice heat resistance[D]. Wuhan: Huangzhong Agricultural University, 2021. (in Chinese with English abstract) | |
| [40] | 刘清泉. 铜胁迫下水稻木质素合成的响应机制及水稻漆酶在植物重金属耐性中的作用[D]. 南京: 南京农业大学, 2015. |
| Liu Q Q. Response mechanism of lignin synthesis in rice under copper stress and the role of rice in plants tolerance to heavy metal[D]. Nanjing: Nanjing Agricultural University, 2015. (in Chinese with English abstract) | |
| [41] | 崔莹莹, 王晓玲. 水稻产量相关性状QTL的遗传研究进展[J]. 江苏农业科学, 2017, 45(13): 1-7. |
| Cui Y Y, Wang X L. Genetic research progress of QTL related traits in rice yield[J]. Jiangsu Agricultural Sciences, 2017, 45(13): 1-7. (in Chinese) | |
| [42] | 张桂莲, 张顺堂, 肖浪涛, 武小金, 肖应辉, 陈立云. 花期高温胁迫对水稻花药生理特性及花粉性状的影响[J]. 作物学报, 2013, 39(1): 177-183. |
| Zhang G L, Zhang S T, Xiao L T, Wu X J, Xiao Y H, Chen L Y. Effect of high temperature stress on physiological characteristics of anther and pollen traits of rice at flowering stage[J]. Acta Agronomica Sinica, 2013, 39(1): 177-183. (in Chinese with English abstract) | |
| [43] | Oshino T, Abiko M, Saito R, Higashitani A. Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high-temperature in barley plants[J]. Molecular Genetics and Genomics, 2007, 278(1): 31-42. |
| [44] | Feng B H, Zhang C X, Chen T T, Zhang X F, Tao L X, Fu G F. Salicylic acid reverses pollen abortion of rice caused by heat stress[J]. BMC Plant Biology, 2018, 18(1): 245. |
| [45] | Fraser C M, Chapple C. The phenylpropanoid pathway in Arabidopsis[J]. Arabidopsis Book, 2011, 9: e0152. |
| [46] | Dixon R A. Natural products and plant disease resistance[J]. Nature, 2001, 411(6839): 843-847. |
| [47] | Mishra A K, Baek K H. Salicylic acid biosynthesis and metabolism: A divergent pathway for plants and bacteria[J]. Biomolecules, 2021. 11(5): 705. |
| [48] | 陈爱国, 彭东, 陈向东, 石屹, 梁洪波. 烤烟苯丙烷代谢中相关酶活性和多酚产物的关系研究[C]. 山东植物生理学会第七次代表大会暨植物生物学与现代农业研讨会, 济南: 山东省科学技术协会, 2012: 240-246. |
| Chen A G, Peng D, Chen X D, Shi Y, Liang H B. Studies on Relationships of Related Enzyme Activities and Polyphenol Products of Phenylpropanoid Metabolic Pathway in Flue-cured tobacco[C]. Proceedings of the 7th Congress of Shandong Plant Physiology Society and Symposium on Plant Biology and Modern Agriculture, Jinan: Shandong Science and technology Association, 2012: 240-246. (in Chinese with English abstract) | |
| [49] | Zhang C X, Feng B H, Chen T T, Fu W M, Li H B, Li G Y, Jin Q Y, Fu G F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation[J]. Environmental and Experimental Botany, 2018, 155: 718-733. |
| [50] | Baena-González E, Hanson J. Shaping plant development through the SnRK1-TOR metabolic regulators[J]. Current Opinion in Plant Biology, 2017, 35: 152-157. |
| [51] | Yu P H, Jiang N, Fu W M, Zheng G J, Li G Y, Feng B H, Chen T T, Ma J Y, Li H B, Tao L X, Fu G F. ATP hydrolysis determines cold tolerance by regulating available energy for glutathione synthesis in rice seedling plants[J]. Rice, 2020, 13(1): 23. |
| [52] | Li G Y, Zhang C X, Zhang G H, Fu G F. Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis[J]. Rice, 2020, 13(1): 18. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 赵艺婷, 谢可冉, 高逖, 崔克辉. 水稻分蘖期干旱锻炼对幼穗分化期高温下穗发育和产量形成的影响[J]. 中国水稻科学, 2024, 38(3): 277-289. |
| [12] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [13] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [14] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [15] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||