
中国水稻科学 ›› 2022, Vol. 36 ›› Issue (6): 623-638.DOI: 10.16819/j.1001-7216.2022.211102
收稿日期:2021-11-02
修回日期:2022-04-05
出版日期:2022-11-10
发布日期:2022-11-10
通讯作者:
蒋冬花
基金资助:
MA Jingjing, PAN Yanyan, YANG Sunyuyue, WANG Jiaqi, JIANG Donghua( )
)
Received:2021-11-02
Revised:2022-04-05
Online:2022-11-10
Published:2022-11-10
Contact:
JIANG Donghua
摘要: 【目的】水稻白叶枯病是水稻的主要细菌性病害之一,严重影响水稻生产。本研究获得一株有效防治水稻白叶枯病的放线菌菌株,探索其对白叶枯病的防效和促进水稻生长的作用,为生防菌的开发利用提供科学依据。【方法】利用梯度稀释涂布法、共培养法和牛津杯法筛选拮抗水稻白叶枯病菌(Xanthomonas oryzae pv. oryzae, Xoo)的放线菌菌株;通过形态特征、生理生化反应、16S rDNA序列和系统发育树分析对其进行分类鉴定;利用共培养法分析目标菌株的抗菌谱;通过96孔板微量稀释法、扫描电子显微镜、微生物粘附碳氢化合物法等探究目标菌株的抑菌作用;通过发酵滤液浸种与菌液喷洒土壤的方法探究其对水稻生长的促进作用;利用盆栽试验探索目标菌株发酵滤液对水稻白叶枯病的防治效果。【结果】从不同生境土壤中分离出140株放线菌菌株,最终筛选得到一株对Xoo具有强拮抗活性的放线菌菌株St-79,其发酵滤液抑菌圈直径为64 mm,并鉴定其为硫藤黄链霉菌(Streptomyces thioluteus)。菌株St-79对水稻细菌性条斑病菌(Xanthomonas oryzae pv. oryzicola)、大豆细菌性斑疹病菌(Xanthomonas axonopodis pv. glycines)、油菜黑腐病菌(Xanthomonas campestris pv. campestris)和番茄细菌性叶斑病菌(Pseudomonas syringae pv. tomato)均有拮抗作用。其乙酸乙酯粗提物具有良好的抑制Xoo生长的效果,粗提物对Xoo的最低抑菌浓度为8 μg/mL,最低杀灭浓度为32 μg/mL;扫描电镜观察结果显示,粗提物对Xoo细胞有很强的损伤作用;粗提物还能改变Xoo细胞内膜通透性,降低Xoo细胞的疏水性。促生试验结果显示,菌株St-79发酵滤液能够促进水稻幼苗的生长。盆栽试验结果显示,菌株St-79发酵滤液对水稻白叶枯病有良好的防治效果,在日本晴、甬优15、甬优1540上的相对防效为65.95%~87.23%,病斑抑制率为69.85%~95.8%,且预防效果优于治疗效果。【结论】获得一株有效防治水稻白叶枯病的放线菌,其对水稻白叶枯病菌有明显的抑制作用,并且可以促进水稻种子的萌发与幼苗的生长。
马静静, 潘妍妍, 杨孙玉悦, 王嘉琦, 蒋冬花. 硫藤黄链霉菌St-79对水稻白叶枯病的防效和促生作用[J]. 中国水稻科学, 2022, 36(6): 623-638.
MA Jingjing, PAN Yanyan, YANG Sunyuyue, WANG Jiaqi, JIANG Donghua. Control Effect of St-79 (Streptomyces thioluteus) on Rice Bacterial Blight and Its Growth-promoting Effect[J]. Chinese Journal OF Rice Science, 2022, 36(6): 623-638.
| 处理组 Treatment group | 处理方法 Treatment method |
|---|---|
| 空白对照组(CK1) Blank control group(CK1) | 无菌剪刀45°剪叶,并套袋保持接种处湿润24 h。下同 Cut leaves with sterile scissors, and bag them to keep the inoculation site moist for for 24 h. The same below. |
| Xoo处理对照组(CK2) Xoo treatment control group(CK2) | 无菌剪刀蘸取Xoo菌液后剪叶 Cut leaves with scissors following a dip into Xoo bacterial liquid. |
| 处理组1(T1) Treatment group 1(T1) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释4倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株),共喷洒3次(1 h喷1次)。下同 Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 4-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant) for a total of 3 sprays (once every hour). The same below. |
| 处理组2(T2) Treatment group 2(T2) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释5倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株) Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 5-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant). |
| 处理组3(T3) Treatment group 3(T3) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释6倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株) Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 6-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant). |
| 处理组4(T4) Treatment group 4(T4) | 无菌剪刀剪叶后立即喷洒稀释4倍的目标菌株发酵滤液(剂量:10 mL/株);6 h后按照CK2的处理方法接种Xoo病菌 Cut leaves with sterile scissors and spray the 4-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant); 6 h post-spraying, inoculate Xoo bacteria according to the CK2 treatment method. |
表1 防效试验设计
Table 1. Experimental design of control effect.
| 处理组 Treatment group | 处理方法 Treatment method |
|---|---|
| 空白对照组(CK1) Blank control group(CK1) | 无菌剪刀45°剪叶,并套袋保持接种处湿润24 h。下同 Cut leaves with sterile scissors, and bag them to keep the inoculation site moist for for 24 h. The same below. |
| Xoo处理对照组(CK2) Xoo treatment control group(CK2) | 无菌剪刀蘸取Xoo菌液后剪叶 Cut leaves with scissors following a dip into Xoo bacterial liquid. |
| 处理组1(T1) Treatment group 1(T1) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释4倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株),共喷洒3次(1 h喷1次)。下同 Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 4-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant) for a total of 3 sprays (once every hour). The same below. |
| 处理组2(T2) Treatment group 2(T2) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释5倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株) Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 5-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant). |
| 处理组3(T3) Treatment group 3(T3) | 按照CK2的处理方法接种Xoo病菌;6 h后用喷壶将稀释6倍的目标菌株发酵滤液均匀地喷洒在伤口处(剂量:10 mL/株) Inoculate Xoo bacteria according to the treatment method of CK2; 6 h later, use a watering can to evenly spray the 6-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant). |
| 处理组4(T4) Treatment group 4(T4) | 无菌剪刀剪叶后立即喷洒稀释4倍的目标菌株发酵滤液(剂量:10 mL/株);6 h后按照CK2的处理方法接种Xoo病菌 Cut leaves with sterile scissors and spray the 4-fold dilution fermentation liquid of the target strain on the wound (dose: 10 mL/plant); 6 h post-spraying, inoculate Xoo bacteria according to the CK2 treatment method. |
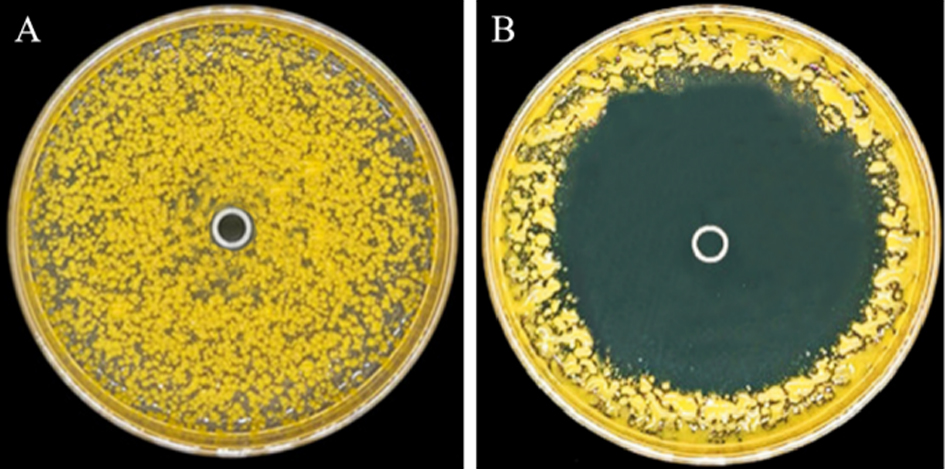
图2 菌株St-79发酵滤液对Xoo的抑菌圈 A-高氏一号液体培养基对照;B-菌株St-79的发酵滤液处理。
Fig. 2. Inhibition zone of fermentation liquid of the strain St-79 against Xoo. A, Control with Kohl’s 1 liquid; B, Treatment with fermentation liquid of strain St-79.
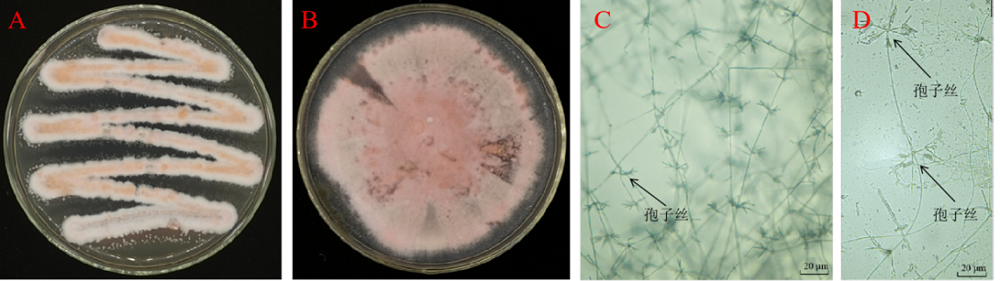
图3 菌株St-79的菌落特征和显微特征 A、B-菌株St-79的菌落形态;C、D-光学显微镜下的气生菌丝体和孢子丝。
Fig. 3. Colony and microscopic observation of strain St-79. A and B, Colonies of strain St-79; C and D, Aerial mycelium and chains of spores under an optical microscope.
| 试验项目 Test item | 试验结果 Reaction | 试验项目 Test items | 试验结果 Reaction |
|---|---|---|---|
| 淀粉水解Hydrolysis of starch | ++ | 硫化氢产生Hydrogen sulfide production | +++ |
| 纤维素水解Hydrolysis of cellulose | ++ | 黑色素产生Melanin production | − |
| 明胶液化Gelatin liquefaction test | +++ | 过氧化氢酶Catalase | +++ |
| MR实验Methyl Red test | − | 脲酶Urease | − |
| V-P实验Voges-Proskauer test | − | 脂肪酶Lipase | + |
表2 菌株St-79生理生化试验结果
Table 2. Physiological and biochemical test results of strain St-79.
| 试验项目 Test item | 试验结果 Reaction | 试验项目 Test items | 试验结果 Reaction |
|---|---|---|---|
| 淀粉水解Hydrolysis of starch | ++ | 硫化氢产生Hydrogen sulfide production | +++ |
| 纤维素水解Hydrolysis of cellulose | ++ | 黑色素产生Melanin production | − |
| 明胶液化Gelatin liquefaction test | +++ | 过氧化氢酶Catalase | +++ |
| MR实验Methyl Red test | − | 脲酶Urease | − |
| V-P实验Voges-Proskauer test | − | 脂肪酶Lipase | + |
| 碳源种类 Type of carbon source | 试验结果 Test result | 氮源种类 Type of nitrogen source | 试验结果 Test result |
|---|---|---|---|
| 葡萄糖Glucose | +++ | 蛋白胨Peptone | +++ |
| 乳糖Lactose | ++ | KNO3 | ++ |
| 麦芽糖Maltose | ++ | (NH4)2SO4 | +++ |
| 木糖Xylose | + | 谷氨酸Glutamate | ++ |
| 蔗糖Sucrose | ++ | 甲硫氨酸Methionine | + |
| 鼠李糖Rhamnose | ++ | 赖氨酸Lysine | +++ |
| 肌醇Inositol | ++ | 亮氨酸Leucine | + |
| 阿拉伯糖L-arabinose | ++ | 组氨酸Histidine | ++ |
| 甘露醇mannitol | +++ |
表3 菌株St-79碳源、氮源利用试验结果
Table 3. Test results of utilization of carbon source and nitrogen source of strain St-79.
| 碳源种类 Type of carbon source | 试验结果 Test result | 氮源种类 Type of nitrogen source | 试验结果 Test result |
|---|---|---|---|
| 葡萄糖Glucose | +++ | 蛋白胨Peptone | +++ |
| 乳糖Lactose | ++ | KNO3 | ++ |
| 麦芽糖Maltose | ++ | (NH4)2SO4 | +++ |
| 木糖Xylose | + | 谷氨酸Glutamate | ++ |
| 蔗糖Sucrose | ++ | 甲硫氨酸Methionine | + |
| 鼠李糖Rhamnose | ++ | 赖氨酸Lysine | +++ |
| 肌醇Inositol | ++ | 亮氨酸Leucine | + |
| 阿拉伯糖L-arabinose | ++ | 组氨酸Histidine | ++ |
| 甘露醇mannitol | +++ |
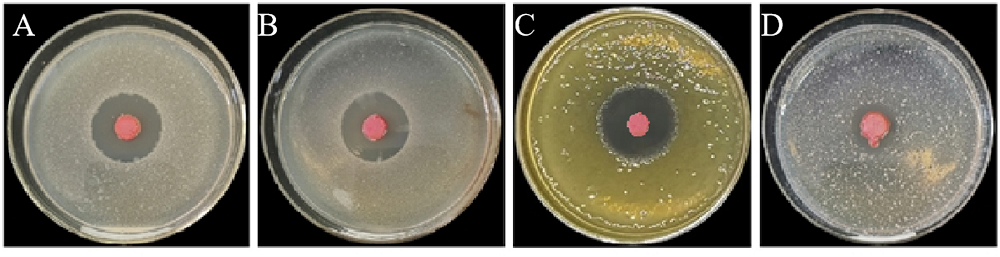
图5 菌株St-79对4种植物病原菌的抑制作用 A-水稻细菌性条斑病菌;B-油菜黑腐病菌;C-大豆细菌性斑疹病菌;D-番茄细菌性叶斑病菌。
Fig. 5. Antibacterial effect of fermentation liquid of strain St-79 against four kinds of plant pathogenic bacteria. A, X. oryzae pv. oryzicola; B, X. campestris pv. campestris; C, X. axonopodis pv. glycines; D, P. syringae pv. tomato.
| 植物病原细菌 Phytopathogenic bacteria | 抑菌圈直径 Inhibition zone diameter /mm |
|---|---|
| 水稻细菌性条斑病菌X. oryzae pv. oryzicola | 34.85±0.49 b |
| 油菜黑腐病菌X. campestris pv. campestris | 33.95±0.35 b |
| 大豆细菌性斑疹病菌X. axonopodis pv. glycines | 32.25±0.70 b |
| 番茄细菌性叶斑病菌P. syringae pv. tomato | 19.95±0.21 a |
表4 菌株St-79对4种植物病原细菌的抑制效果
Table 4. Inhibition zone of fermentation liquid from strain St-79 against four kinds of plant pathogenic bacteria.
| 植物病原细菌 Phytopathogenic bacteria | 抑菌圈直径 Inhibition zone diameter /mm |
|---|---|
| 水稻细菌性条斑病菌X. oryzae pv. oryzicola | 34.85±0.49 b |
| 油菜黑腐病菌X. campestris pv. campestris | 33.95±0.35 b |
| 大豆细菌性斑疹病菌X. axonopodis pv. glycines | 32.25±0.70 b |
| 番茄细菌性叶斑病菌P. syringae pv. tomato | 19.95±0.21 a |
| 菌株St-79乙酸乙酯粗提物浓度 Concentration of crude extracts of strain St-79/(μg·mL−1) | 粗提物作用24 h后培养液浊度 Turbidity of culture medium after treatment with crude extracts for 24 h | 平板上菌体生长情况 Growth of bacteria on the plate |
|---|---|---|
| 0 | 浑浊Muddy | 有菌生长With Xoo |
| 2 | 浑浊Muddy | 有菌生长With Xoo |
| 4 | 浑浊Muddy | 有菌生长With Xoo |
| 8 | 澄清Clarification | 有菌生长With Xoo |
| 16 | 澄清Clarification | 有菌生长With Xoo |
| 32 | 澄清Clarification | 无菌生长Without Xoo |
| 64 | 澄清Clarification | 无菌生长Without Xoo |
| 128 | 澄清Clarification | 无菌生长Without Xoo |
| 256 | 澄清Clarification | 无菌生长Without Xoo |
表5 菌株St-79粗提物对Xoo的MIC和MBC
Table 5. MIC and MBC of crude extracts of target strain against Xoo.
| 菌株St-79乙酸乙酯粗提物浓度 Concentration of crude extracts of strain St-79/(μg·mL−1) | 粗提物作用24 h后培养液浊度 Turbidity of culture medium after treatment with crude extracts for 24 h | 平板上菌体生长情况 Growth of bacteria on the plate |
|---|---|---|
| 0 | 浑浊Muddy | 有菌生长With Xoo |
| 2 | 浑浊Muddy | 有菌生长With Xoo |
| 4 | 浑浊Muddy | 有菌生长With Xoo |
| 8 | 澄清Clarification | 有菌生长With Xoo |
| 16 | 澄清Clarification | 有菌生长With Xoo |
| 32 | 澄清Clarification | 无菌生长Without Xoo |
| 64 | 澄清Clarification | 无菌生长Without Xoo |
| 128 | 澄清Clarification | 无菌生长Without Xoo |
| 256 | 澄清Clarification | 无菌生长Without Xoo |
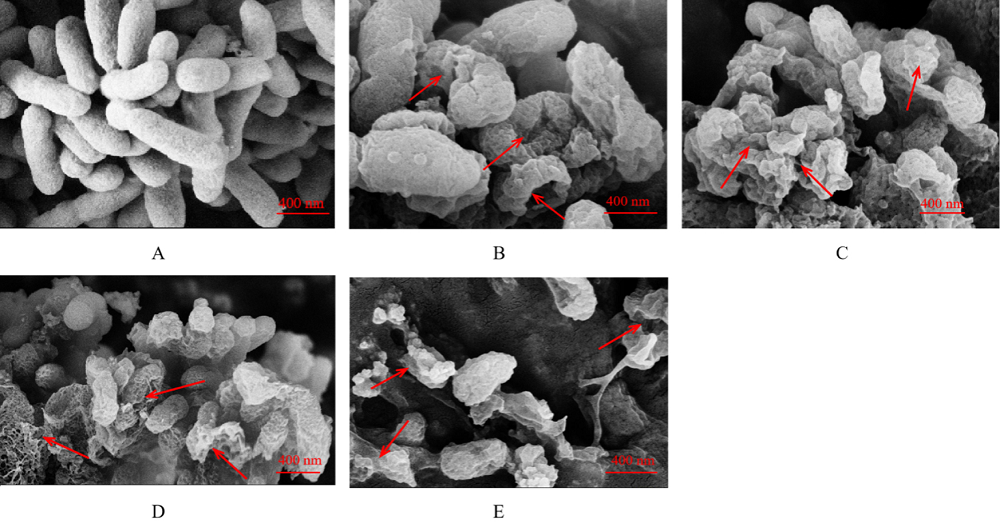
图7 不同浓度粗提物处理4 h后Xoo细胞扫描电子显微镜观察结果 A-对照;B-4 μg/mL;C-8 μg/mL;D-16 μg/mL;E-32 μg/mL。
Fig. 7. Xoo cells treated with crude extracts at different concentrations for 4 h under a scanning electron microscopy. A, Control; B, 4 μg/mL; C, 8 μg/mL; D, 16 μg/mL; E, 32 μg/mL.
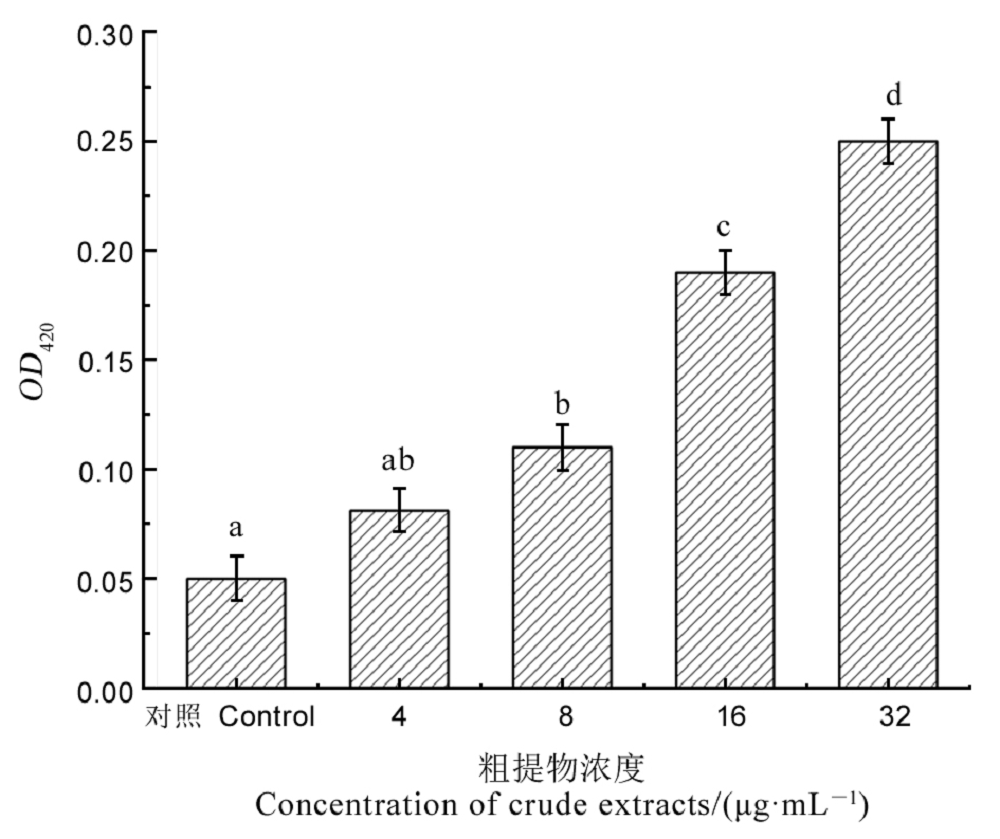
图8 粗提物处理对Xoo细胞内膜通透性的影响 不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 8. Intimal permeability of Xoo cells treated with crude extracts. Different letters indicate significant difference at the P < 0.05 level.
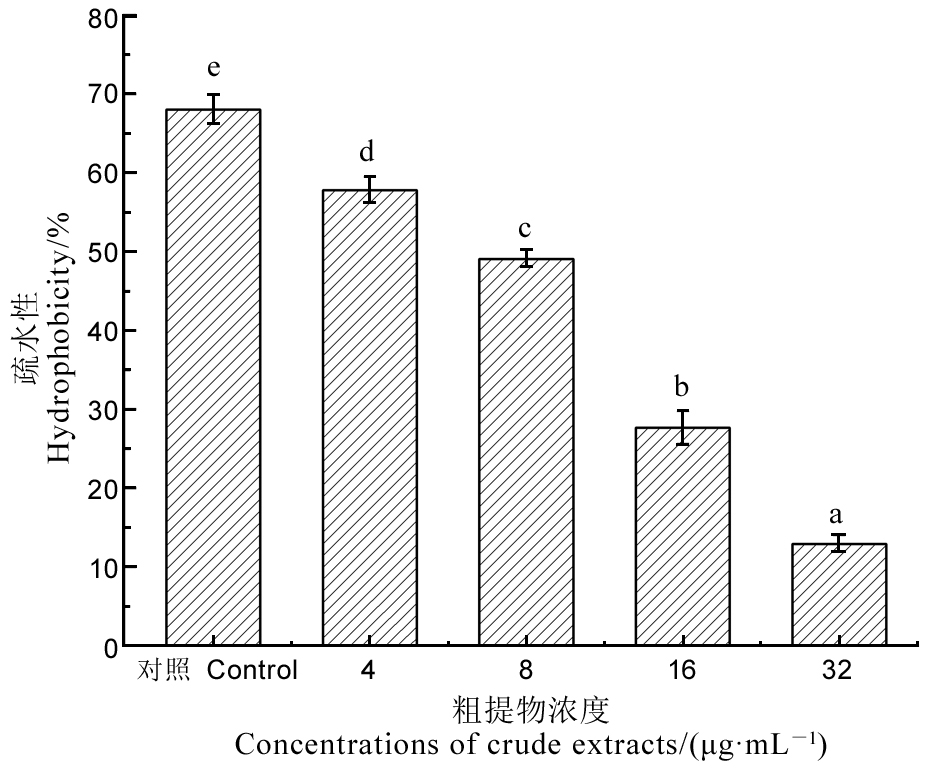
图9 不同浓度粗提物处理4 h后对Xoo表面疏水性的影响 不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 9. Hydrophobicity assay of Xoo treated with different concentrations of crude extracts for 4 h. Different letters indicate significant difference at the P < 0.05 level.
| 处理 Treatment | 处理后天数Days after treatment/d | ||
|---|---|---|---|
| 4 | 6 | 8 | |
| 发酵滤液原液Crude fermentation liquid | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| 高氏1号液体培养基原液 Crude Gauze’s medium No. 1 | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| 发酵滤液10倍稀释液1/10 fermentation liquid | 25.33±3.06 b | 63.33±1.15 b | 72.67±1.15 c |
| 高氏1号液体培养基10倍稀释液1/10 Gauze’s medium No. 1 | 23.00±3.00 b | 59.33±1.15 b | 66.00±2.00 b |
| 发酵滤液50倍稀释液1/50 fermentation liquid | 32.00±3.46 cd | 72.00±2.00 c | 78.00±2.00 d |
| 高氏1号液体培养基50倍稀释液1/50 Gauze’s medium No. 1 | 28.67±3.06 bc | 70.67±1.15 c | 76.67±3.06 d |
| 发酵滤液100倍稀释液1/100 fermentation liquid | 53.33±3.06 e | 91.33±2.31 f | 96.67±1.15 g |
| 高氏1号液体培养基100倍稀释液1/100 Gauze’s medium No. 1 | 29.33±1.15 bc | 79.33±1.15 de | 87.00±1.00 f |
| 发酵滤液500倍稀释液1/500 fermentation liquid | 39.33±1.15 d | 82.67±2.31 e | 84.67±1.15 ef |
| 高氏1号液体培养基500倍稀释液1/500 Gauze’s medium No. 1 | 30.67±3.06 bc | 76.00±2.00 cd | 80.67±1.15 de |
| 无菌水H2O | 30.00±2.00 bc | 80.67±1.15 de | 88.67±1.15 f |
表6 发酵滤液对水稻种子萌发率的影响
Table 6. Effect of fermentation liquid of St-79 on rice seed germination percentage. %
| 处理 Treatment | 处理后天数Days after treatment/d | ||
|---|---|---|---|
| 4 | 6 | 8 | |
| 发酵滤液原液Crude fermentation liquid | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| 高氏1号液体培养基原液 Crude Gauze’s medium No. 1 | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| 发酵滤液10倍稀释液1/10 fermentation liquid | 25.33±3.06 b | 63.33±1.15 b | 72.67±1.15 c |
| 高氏1号液体培养基10倍稀释液1/10 Gauze’s medium No. 1 | 23.00±3.00 b | 59.33±1.15 b | 66.00±2.00 b |
| 发酵滤液50倍稀释液1/50 fermentation liquid | 32.00±3.46 cd | 72.00±2.00 c | 78.00±2.00 d |
| 高氏1号液体培养基50倍稀释液1/50 Gauze’s medium No. 1 | 28.67±3.06 bc | 70.67±1.15 c | 76.67±3.06 d |
| 发酵滤液100倍稀释液1/100 fermentation liquid | 53.33±3.06 e | 91.33±2.31 f | 96.67±1.15 g |
| 高氏1号液体培养基100倍稀释液1/100 Gauze’s medium No. 1 | 29.33±1.15 bc | 79.33±1.15 de | 87.00±1.00 f |
| 发酵滤液500倍稀释液1/500 fermentation liquid | 39.33±1.15 d | 82.67±2.31 e | 84.67±1.15 ef |
| 高氏1号液体培养基500倍稀释液1/500 Gauze’s medium No. 1 | 30.67±3.06 bc | 76.00±2.00 cd | 80.67±1.15 de |
| 无菌水H2O | 30.00±2.00 bc | 80.67±1.15 de | 88.67±1.15 f |
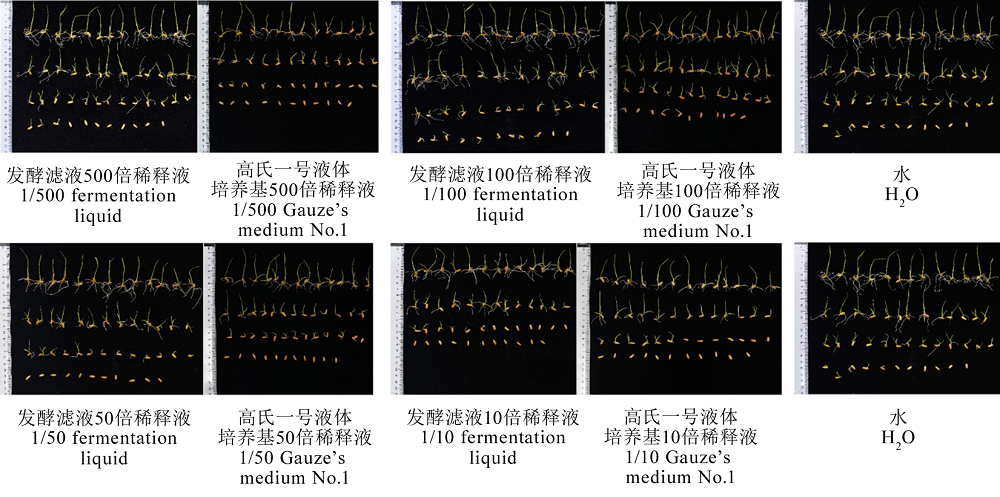
图10 发酵滤液浸种8 d后的水稻种子发芽情况 不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 10. Germination of rice seeds soaked in fermentation liquid for 8 days. Different letters indicate significant differences at the P < 0.05 level.
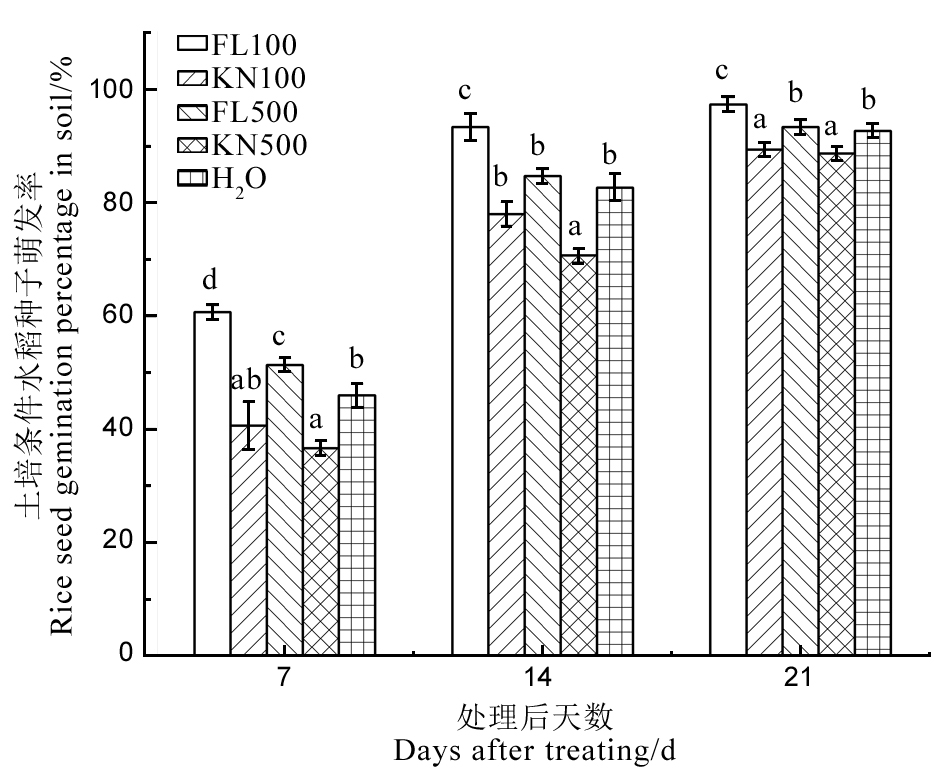
图11 土培条件下菌株St-79发酵滤液对水稻种子萌发率的影响 FL100、FL500分别表示菌株St-79发酵滤液稀释100倍和500倍;KN100、KN500分别表示高氏1号液体培养基稀释100倍和500倍,下同。不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 11. Effect of fermentation liquid of strain St-79 on rice seed germination percentage in soil. FL100, FL500 indicate 1/100 and 1/500 fermentation liquid of strain St-79 respectively; KN100, KN500 indicate 1/100 and 1/500 Gauze’s medium No. 1 respectively. The same below. Different letters indicate significant differences at the P < 0.05 level.
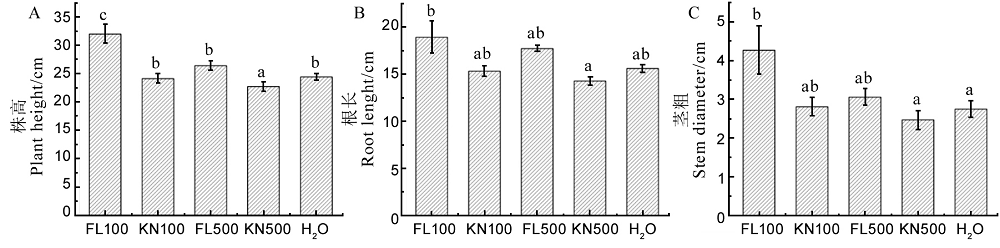
图13 菌株St-79发酵滤液对水稻幼苗株高、根长和茎粗的影响 A-株高;B-根长;C-茎粗。不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 13. Effects of fermentation liquid of strain St-79 on plant height, root length and stem diameter of rice seedlings. A, Plant height; B, Root length; C, Stem diameter. Different letters indicate significant differences at the P < 0.05 level.
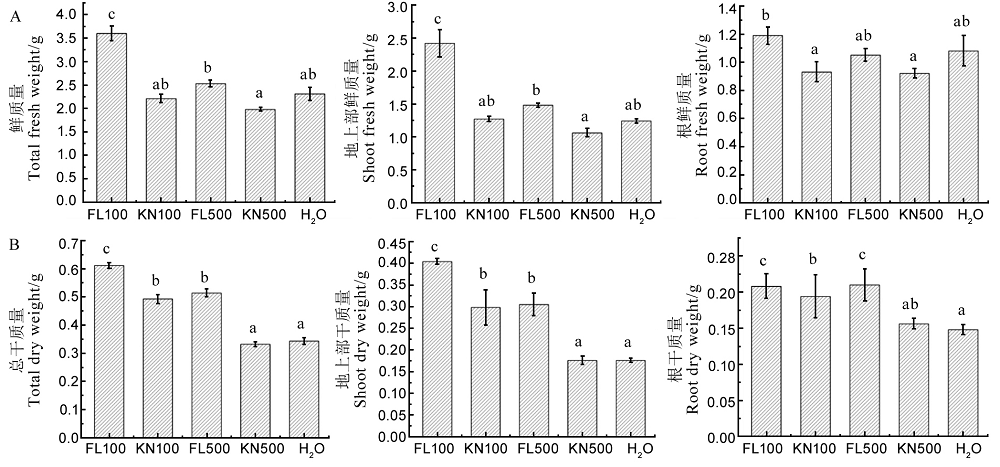
图14 菌株St-79发酵滤液对水稻幼苗鲜质量和干质量的影响 A-菌株St-79发酵滤液对水稻幼苗总鲜质量、地上部鲜质量和根鲜质量的影响;B-菌株St-79发酵滤液对水稻幼苗总干质量、地上部干质量、根干质量的影响。不同小写字母表示在P < 0.05水平存在显著差异。
Fig. 14. Effects of fermentation liquid of strain St-79 on wet weight and dry weight of rice seedlings. A, Effects of fermentation liquid of strain St-79 on fresh weight, shoot fresh weight and root fresh weight of rice seedlings; B, Effects of fermentation liquid of strain St-79 on dry weight, shoot dry weight and root dry weight of rice seedlings. Different letters indicate significant differences at the P < 0.05 level.
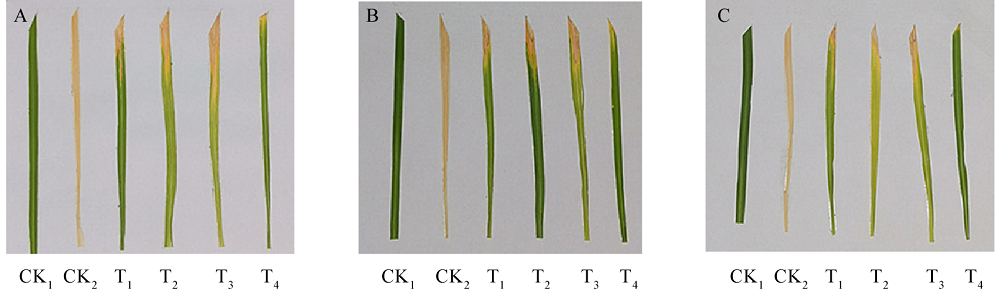
图15 不同处理下水稻品种病斑长度 A-甬优1540;B-甬优15;C-日本晴。CK1-空白对照;CK2-只接种Xoo;T1-接种Xoo 6 h后,喷洒稀释4倍的发酵滤液;T2-接种Xoo 6 h后,喷撒稀释5倍的发酵滤液;T3-接种Xoo 6 h后,喷撒稀释6倍的发酵滤液;T4-剪叶后喷洒稀释4倍的发酵滤液,6 h后接种Xoo。下同。
Fig. 15. Lesion length of rice varieties under different treatments. A, Yongyou 1540; B, Yongyou 15; C, Nipponbare. CK1, Blank control; CK2, Control with inoculating Xoo only; T1, Inoculation with Xoo before treatment with 4-fold dilution fermentation liquid; T2, Inoculation with Xoo before treatment with 5-fold dilution fermentation liquid; T3, Inoculation with Xoo before treatment with 6-fold dilution fermentation liquid; T4, Treatment with 4-fold dilution fermentation liquid before inoculation with Xoo, the same blow.
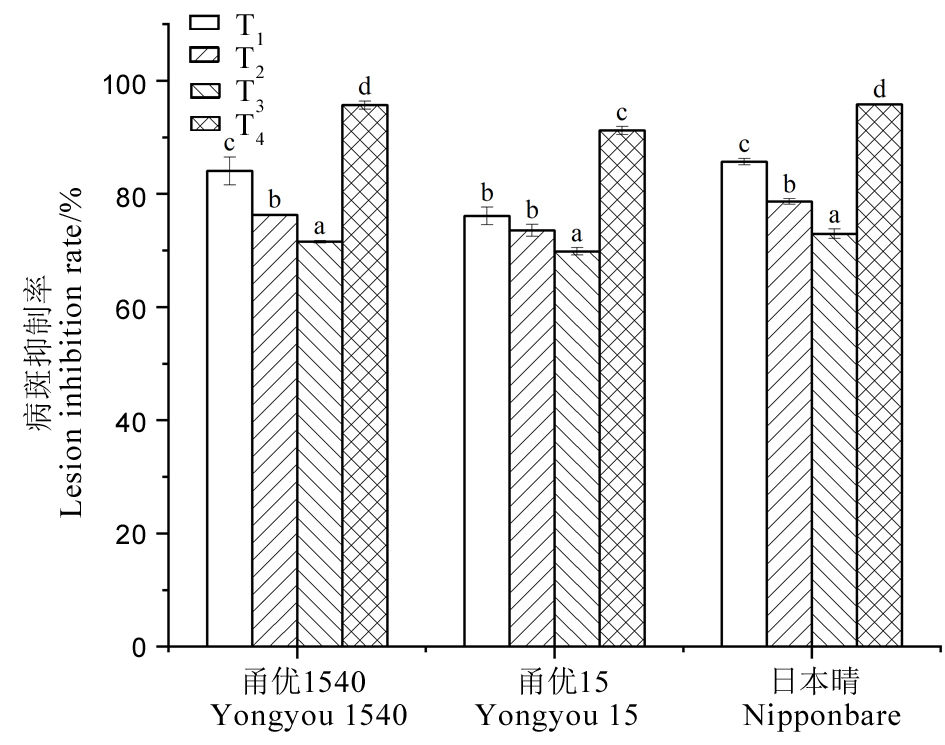
图16 不同处理下水稻品种病斑抑制率 同一品种标相同小写字母者表示在P < 0.05水平存在显著差异。
Fig. 16. Lesion inhibition rate of rice varieties with different treatments For a variety, common letters indicate no significant difference at the P < 0.05 level.
| 处理 Treatment | 病情指数Disease index | 相对防效Relative control effect/% | |||||
|---|---|---|---|---|---|---|---|
| 甬优1540 Yongyou 1540 | 甬优15 Yongyou 15 | 日本晴 Nipponbare | 甬优1540 Yongyou 1540 | 甬优15 Yongyou 15 | 日本晴 Nipponbare | ||
| CK1 | 0 | 0 | 0 | / | / | / | |
| CK2 | 95.66±0.29 e | 95.93±0.52 e | 97.41±0.52 e | 0 | 0 | 0 | |
| T1 | 16.93±0.42 b | 22.02±0.55 b | 18.16±1.06 b | 82.31±0.29 c | 77.05±0.70 c | 81.35±1.19 c | |
| T2 | 23.13±1.05 c | 27.40±1.59 c | 23.19±0.84 c | 75.84±0.86 b | 71.23±1.53 b | 76.20±1.00 b | |
| T3 | 30.93±0.26 d | 32.76±0.24 d | 27.98±0.24 d | 67.67±0.55 a | 65.85±0.07 a | 71.28±0.09 a | |
| T4 | 12.23±1.58 a | 15.00±0.79 a | 14.07±0.52 a | 87.23±1.53 d | 84.38±0.74 d | 85.56±0.62 d | |
表7 不同处理对3个水稻品种的病情指数及相对防效的影响
Table 7. Disease index and relative control effect on three rice varieties under different treatments.
| 处理 Treatment | 病情指数Disease index | 相对防效Relative control effect/% | |||||
|---|---|---|---|---|---|---|---|
| 甬优1540 Yongyou 1540 | 甬优15 Yongyou 15 | 日本晴 Nipponbare | 甬优1540 Yongyou 1540 | 甬优15 Yongyou 15 | 日本晴 Nipponbare | ||
| CK1 | 0 | 0 | 0 | / | / | / | |
| CK2 | 95.66±0.29 e | 95.93±0.52 e | 97.41±0.52 e | 0 | 0 | 0 | |
| T1 | 16.93±0.42 b | 22.02±0.55 b | 18.16±1.06 b | 82.31±0.29 c | 77.05±0.70 c | 81.35±1.19 c | |
| T2 | 23.13±1.05 c | 27.40±1.59 c | 23.19±0.84 c | 75.84±0.86 b | 71.23±1.53 b | 76.20±1.00 b | |
| T3 | 30.93±0.26 d | 32.76±0.24 d | 27.98±0.24 d | 67.67±0.55 a | 65.85±0.07 a | 71.28±0.09 a | |
| T4 | 12.23±1.58 a | 15.00±0.79 a | 14.07±0.52 a | 87.23±1.53 d | 84.38±0.74 d | 85.56±0.62 d | |
| [1] | 刘琴英. 高效拮抗水稻白叶枯病菌植物内生真菌的选育和防效研究[D]. 金华: 浙江师范大学, 2014. |
| Liu Q Y. Breeding and Biocontrol Effects of Endophytic fungi with high antimicrobial activity to Xanthomonas oryzae pv. oryzae[D]. Jinhua: Zhejiang Normal University, 2014. (in Chinese with English abstract) | |
| [2] | 高杜娟, 唐善军, 陈友德, 李友荣. 近30年国家和湖南省审定的水稻品种白叶枯病抗性分析[J]. 中国稻米, 2017, 23(1): 65-68. |
| Gao D J, Tang S J, Chen Y D, LI Y R. Bacterial blight resistance analysis of China national and Hunan Province authorized rice varieties in recent 30 years[J]. China Rice, 2017, 23(1): 65-68. (in Chinese with English abstract) | |
| [3] | 刘永锋, 陆凡, 陈志谊, 张芬, 聂亚锋, 于俊杰, 尹小乐. 拮抗细菌T429和T392的生物活性及其对水稻白叶枯病的防治效果[J]. 江苏农业学报, 2012, 28(4): 733-737. |
| Liu Y F, Lu F, Cheng Z Y, Zhang F, Nie Y F, Yu J J, Yin X L. Biological activities of antagonistic bacterial isolates T429 and T392 and their control effects against rice bacterial leaf blight (Xanthomonas oryzae)[J]. Jiangsu Journal of Agricultural Sciences, 2012, 28(4): 733-737. (in Chinese with English abstract) | |
| [4] | Chen Y, Yang X, Gu C Y, Zhang A F, Zhang Y, Wang W X, Gao T C, Yao J, Yuan S K. Activity of a novel bactericide, zinc thiazole against Xanthomonas oryzae pv. oryzae in Anhui Province of China[J]. Annals of Applied Biology, 2015, 166(1): 129-135. |
| [5] | Chen W J, Kuo T Y, Chen C Y, Hsieh F C, Yang Y L, Liu J R, Shih M C. Whole genome sequencing and Tn5-Insertion mutagenesis of Pseudomonas taiwanens CMS to probe its antagonistic activity against rice bacterial blight disease[J]. International Journal of Molecular Sciences, 2020, 21(22): 8639. |
| [6] | Liu C X, Zhang J, Wang X J, Qian P T, Wang J D, Gao Y M, Yan Y J, Zhang S Z, Xu P F, Li W B, Xiang W S. Antifungal activity of borrelidin produced by a Streptomyces strain isolated from soybean[J]. Journal of Agricultural and Food Chemistry, 2012, 60(5): 1251-1257. |
| [7] | Shen J F, Kong L X, Li Y, Zheng X Q, Wang Q, Yang W N, Deng Z X, You D L. A LuxR family transcriptional regulator AniF promotes the production of anisomycin and its derivatives in Streptomyces hygrospinosus var. beijingensis[J]. Synthetic and Systems Biotechnology, 2019, 4(1): 40-48. |
| [8] | Li P Q, Feng B Z, Li X X, Hao H Y. Screening and identification of antagonistic actinomycete LA-5 against Botrytis cinerea[J]. Journal of Applied Ecology, 2018, 29(12): 4172-4180. |
| [9] | Yang Y, Zhang S W, Li K T. Antagonistic activity and mechanism of an isolated Streptomyces corchorusii stain AUH-1 against phytopathogenic fungi[J]. World Journal Microbiology Biotechnology, 2019, 35(9): 145. |
| [10] | 王炫栋, 杨孙玉悦, 高润杰, 余俊杰, 郑丹沛, 倪峰, 蒋冬花. 拮抗大豆斑疹病菌放线菌菌株的筛选和促生作用及防效研究[J/OL]. 作物学报: 1-13. |
| Wang X D, Yang S Y Y, Gao R J, Yu J J, Zheng D P, Ni F, Jiang D H. Screening Streptomyces against Xanthomonas axonopodis pv. glycines and study of growth-promoting and biocontrol effect[J/OL]. Acta Agronomica Sinica: 1-13. (in Chinese with English abstract) | |
| [11] | Park S B, Lee I A, Suh J W, Kim J G, Lee C H. Screening and identification of antimicrobial compounds from Streptomyces bottropensis suppressing rice bacterial blight[J]. Journal of Microbiology Biotechnology, 2011, 21(12): 1236-1242. |
| [12] | 张艳军, 张萍华. 水稻白叶枯病拮抗菌的筛选、鉴定及发酵液稳定性分析[J]. 浙江师范大学学报: 自然科学版, 2014, 37(4): 382-387. |
| Zhang Y J, Zhang P H. Screening and identification of antagonistic microorganism against Xanthomonas oryzae and stability analysis of the fermentation liquid[J]. Journal of Zhejiang Normal University: Natural Science Edition, 2014, 37(4): 382-387. (in Chinese with English abstract) | |
| [13] | 史婷婷, 马静静, 郭鑫, 倪峰, 蒋冬花. 拮抗水稻白叶枯病菌链霉菌的筛选、鉴定和防效研究[J]. 植物病理学报, 2021, 51(3): 403-412. |
| Shi T T, Ma J J, Guo X, Ni F, Jiang D H. Screening, identification and greenhouse biological control activity of Streptomyces sp. activity against Xanthomonas oryzae pv. oryzae[J]. Plant Pathology, 2021, 51(3): 403-412. (in Chinese with English abstract) | |
| [14] | Anwar S, Ali B, Sajid I. Screening of rhizospheric actinomycetes for various In-vitro and In-vivo plant growth promoting (PGP) traits and for agroactive compounds[J]. Frontiers in Microbiology, 2016, 7: 1334. |
| [15] | Passari A K, Chandra P, Zothanpuia, Mishra V K, Leo V V, Gupta V K, Kumar B, Singh B P. Detection of biosynthetic gene and phytohormone production by endophytic actinobacteria associated with Solanum lycopersicum and their plant-growth-promoting effect[J]. Research in Microbiology, 2016, 167(8): 692-705. |
| [16] | Lin L, Xu X. Indole-3-acetic acid production by endophytic Streptomyces sp. En-1 isolated from medicinal plants[J]. Current Microbiology, 2013, 67(2): 209-217. |
| [17] | 刘雨晴. 水稻内生放线菌OsiSh-10对植物生长的影响及机理的初探[D]. 长沙: 湖南大学, 2017. |
| Liu Y Q. The effect of endophytic actinomycetes OsiSh-10 on its host plant growth and preliminary study on the mechanism[D]. Changsha: Hunan University, 2017. (in Chinese with English abstract) | |
| [18] | 付学鹏, 沈童飞, 孙晓波, 刘晓涵, 杨晓杰. 链霉菌株Streptomyces sp. FXP04对水稻种子萌发和幼苗生长的影响[J]. 作物杂志, 2020(6): 163-169. |
| Fu X P, Shen T F, Sun X B, Liu X H, Yang X J. Effects of Streptomyces sp. FXP04 on seed germination and seedling growth of rice[J]. Crop Journal, 2020(6): 163-169. (in Chinese with English abstract) | |
| [19] | 张鸿雁, 高擎, 张琳园, 林国莉, 李如莲. 大豆疫病拮抗菌的筛选及促生抗病作用研究[J]. 生物技术通报, 2020, 36(10): 25-31. |
| Zhang H Y, Gao P, Zhang L Y, Lin G L, Li R L. Screening of actinomycetes against Phytophthora root rot of soybean and its growth promotion and disease control[J]. Biotechnology Bulletin, 2020, 36(10): 25-31. (in Chinese with English abstract) | |
| [20] | Jiang B, Wang Z Y, Xu C X, Liu W J, Jiang D H. Screening and identification of Aspergillus activity against Xanthomonas oryzae pv. oryzae and analysis of antimicrobial components[J]. Journal of Microbiology, 2019, 57(7): 597-605. |
| [21] | 胡凌鸣, 徐春毅, 罗嘉琪, 张婧婧, 陆洁俐, 何智鹏, 蒋冬花. 一株拮抗水稻白叶枯病菌的淡紫灰链霉菌的筛选鉴定及其生防效果研究[J]. 植物病理学报, 2022, 52(2): 223-234. |
| Hu L M, Xu C Y, Luo J Q, Zhang J J, Lu J L, He Z P, Jiang D H. Screening, identification and biocontrol effect of a strain of Streptomyces lavendulae against Xanthomonas oryzae pv. oryzae[J/OL]. Acta Phytopathologica Sinica, 2022, 52(2): 223-234. (in Chinese with English abstract) | |
| [22] | 甘良, 蓝星杰, 戴蓬博, 刘继红, 王阳, 宗兆锋. 放线菌混合菌剂对西瓜枯萎病的防治作用研究[J]. 中国生物防治学报, 2015, 31(4): 516-523. |
| Gan L, Lan X J, Dai P B, Liu J H, Wang Y, Zong Z F. Effect of combined biocontrol actinomycetes strains on watermelon wilt disease[J]. Chinese Journal of Biological Control, 2015, 31(4): 516-523. (in Chinese with English abstract) | |
| [23] | Oliveira L C, Silveira A M M, Monteiro A S, Dos Santos V L, Nicoli J R, Azevedo V A C, Soares S C, Dias-Souza M V, Nardi R M D. In silico prediction, in vitro antibacterial spectrum, and physicochemical properties of a putative bacteriocin produced by Lactobacillus rhamnosus strain L156.4[J]. Frontiers in Microbiology, 2017, 8: 876-891. |
| [24] | 沈萍, 陈向东. 微生物学实验. 4版. 北京: 高等教育出版社, 2007. |
| Shen P, Chen X D. Microbiology experiment. 4th ed. Beijing: Higher Education Press, 2007. (in Chinese) | |
| [25] | Shuji O, Masaru A, Hiroshi K. Analysis of antarctic soil algae by the direct observation using the contact slide method[J]. Antarctic Record, 1991, 35(3): 285-295. |
| [26] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册. 2版. 北京: 科学出版社, 2001: 364-399. |
| Dong X Z, Cai M Y. Identification manual for common bacterial systems. 2nd ed. Beijing: Science Press, 2001: 364-399. (in Chinese with English abstract) | |
| [27] | Shirling E B, Gottlieb D. Methods for characterization of Streptomyces species[J]. Intenational Journal of Systematic Bacteriology, 1966, 16(3): 313-340. |
| [28] | 杨铭. 海洋放线菌分离及Streptomyces sp. S42中天然产物的挖掘[D]. 济南: 山东大学, 2020. |
| Yang M. Isolation of marine actinomycetes and natural products exploration from Streptomyces sp. S42s[D]. Jinan: Shandong University, 2020. (in Chinese with English abstract) | |
| [29] | Cho E, Kwon O S, Chung B, Lee J, Sun J, Shin J, Oh K B. Antibacterial activity of chromomycins from a marine-derived Streptomyces microflavus[J]. Marine Drugs, 2020, 18(10): 522. |
| [30] | 黎芳靖. 小檗碱对水稻细菌性条斑病菌的抑菌机制分析[D]. 南宁: 广西大学, 2018. |
| Li F J. Analysis of antibacterial mechanism of berberine against Xanthomonas oryzae pv. oryzicola[D]. Nanjing: Guangxi University, 2018. (in Chinese with English abstract) | |
| [31] | Rosenberg M. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH[J]. FEMS Microbiology Letters, 2006, 262(2): 129-134. |
| [32] | 农业部农药检定所生测室. 农药田间药效试验准则[M]. 北京: 中国标准出版社, 2000: 166-169. |
| Department of Pesticide Testing, Ministry of Agriculture. Guidelines for Field Efficacy Trials of Pesticides[M]. Beijing: China Standard Press, 2000: 166-169. (in Chinese) | |
| [33] | Wang J J, Zhao Y, Ruan Y Z. Effects of bio-organic fertilizers produced by four Bacillus amyloliquefaciens strains on Banana Fusarium wilt disease[J]. Compost Science & Utilization, 2015, 23(3): 185-198. |
| [34] | Kim K R, Kim T J, Suh J W. The gene cluster for Spectinomycin biosynthesis and the aminoglycoside-resistance function of spcM in Streptomyces spectabilis[J]. Current Microbiology, 2008, 57(4): 371-374. |
| [35] | Fukunaga K, Misato T, Ishii I. A new anti-phytopathogenic fungal substance Part I[J]. Bulletin of the Agricultural Chemical Society of Japan, 1955, 19(3): 181-188. |
| [36] | Shi T T, Guo X, Zhu J L, Hu L M, He Z P, Jiang D H. Inhibitory effects of Carbazomycin B produced by Streptomyces roseoverticillatus 63 against Xanthomonas oryzae pv. oryzae[J]. Frontiers in Microbiology, 2021, 12: 616937. |
| [37] | Kim B S, Moon S S, Hwang B K. Isolation, identification, and antifungal activity of a macrolide antibiotic, oligomycin A, produced by Streptomyces libani[J]. Botany, 1999, 77(6): 850-858. |
| [38] | Yang P W, Li M G, Zhao J Y, Zhu M Z, Shang H, Li J R, Cui X L, Huang R, Wen M L. Oligomycins A and C, major secondary metabolites isolated from the newly isolated strain Streptomyces diastaticus[J]. Folia Microbiologica, 2010, 55(1): 10-16. |
| [39] | Hamedi J, Mohammadipanah F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria[J]. Journal of Industrial Microbiology and Biotechnology, 2015, 42(2): 157-171. |
| [40] | Qin S, Feng W W, Wang T T, Ding P, Xing K & Jiang J H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense[J]. Plant and Soil, 2017, 416(1/2): 117-132. |
| [41] | Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney R K. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea[J]. Brazilian Journal of Microbiology, 2016, 47(1): 85-95. |
| [42] | Rungin S, Indananda C, Suttiviriya P, Kruasuwan W, Jaemsaeng R, Thamchaipenet A. Plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105)[J]. Antonie Van Leeuwenhoek, 2012, 2(3): 463-472. |
| [43] | Gopalakrishnan S, Vadlamudi S, Bandikinda P, Sathya A, Vijayabharathi R, Rupela O, Kudapa H, Katta K, Varshney R K. Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice[J]. Microbiological Research, 2014, 169(1): 40-48. |
| [44] | 邹路路, 余雷, 凃昌, 向慧龙, 朱洁倩, 傅本重. 孝顺竹内生菌BME17对水稻白叶枯和条斑病生防效果的初步研究[J]. 湖北大学学报: 自然科学版, 2019, 41(6): 578-583. |
| Zou L L, Yu L, Tu C, Xiang H L, Zhu J Q, Fu B Z. Preliminary study on the biocontrol effect of endophytic bacteria BME17 isolated from Bambusa multiplex on rice bacterial blight and stripe blight[J]. Journal of Hubei University: Natural Science Edition, 2019, 41(6): 578-583. (in Chinese with English abstract) | |
| [45] | 魏兰芳, 周丽洪, 姬广海, 王永吉, 汪绍雪. Lysobacter antibioticus 13-1菌株抗菌物质鉴定及对水稻白叶枯病的防治效果[J]. 微生物学通报, 2014, 41(2): 274-280. |
| Wei L F, Zhou L H, Ji G H, Wang Y J, Wang S X. Control of rice bacterial leaf blight by antibacterial substances from Lysobacter antibioticus strain13-1[J]. Microbiology China, 2014, 41(2): 274-280. (in Chinese with English abstract) | |
| [46] | 罗吉. 荞麦立枯病生防菌的筛选鉴定及生防效果初探[D]. 成都: 成都大学, 2021. |
| Luo J. Screening and identification of biocontrol bacteria and preliminary study on biocontrol effect of buckwheat rhizobia[D]. Chengdu: Chengdu University, 2021. (in Chinese with English abstract) | |
| [47] | 戴秀华, 张荣胜, 陈志谊. 解淀粉芽胞杆菌Lx-11生物学特性研究[J]. 中国生物防治学报, 2014, 30(4): 573-580. |
| Dai X H, Zhang R S, Chen Z Y. The biological characterization of Bacillus amyloliquefaciens Lx-11[J]. Chinese Journal of Biological Control, 2014, 30(4): 573-580. (in Chinese with English abstract) | |
| [48] | 林吉恒. 食品级安全微生物枯草芽孢杆菌LJH25的生物防治作用及机理初探[D]. 杭州: 浙江大学, 2011. |
| Lin J H. Biological control and its mechanism of food-grade Bacillus subtilis LJH25[D]. Hangzhou: Zhejiang University, 2011. (in Chinese with English abstract) | |
| [49] | 李娟. 水稻内生拮抗细菌抗菌蛋白的提取、抗生作用及理化特性[D]. 扬州: 扬州大学, 2007. |
| Li J. Isolation, antibiosis, physical and chemical features of antifungal protein from rice endophytic antagonistic bacterium Bacillus subtilis[D]. Yangzhou: Yangzhou University, 2007. (in Chinese with English abstract) | |
| [50] | 邵正英, 聂丽, 徐志荣, 李张, 傅雁辉, 魏赛金. 链霉菌JD211对水稻酚类物质及相关酶活的影响[J]. 江西农业大学学报, 2017, 39(5): 983-988. |
| Shao Z Y, Nie L, Xu Z R, Li Z, Fu Y H, Wei S J. Effects of Streptomyces JD211 on phenolic compounds and the activity of related enzymes in rice[J]. Acta Agriculturae Universitatis Jiangxiensis, 2017, 39(5): 983-988. (in Chinese with English abstract) |
| [1] | 王炫栋, 余俊杰, 高润杰, 兰赫婷, 江樱姿, 齐文杰, 宋振, 蒋冬花. 一株兼具防病促生功能的沙阿霉素链霉菌Sz-11[J]. 中国水稻科学, 2023, 37(2): 200-212. |
| [2] | 李丽, 莫旭艳, 李甜甜, 张丽媛, 董汉松. 白叶枯病菌效应子XopN在拥有OsSWEET11同源基因的水稻品种上发挥毒性作用[J]. 中国水稻科学, 2020, 34(4): 368-382. |
| [3] | 张清霞, 张迎, 何玲玲, 陈夕军, 童蕴慧, 纪兆林. 水稻纹枯病拮抗细菌7-5的鉴定及其生防机制初步研究[J]. 中国水稻科学, 2018, 32(3): 277-284. |
| [4] | 朱引引,刘永庭,李士河,宋从凤*. 水稻白叶枯菌OS198中talR26.5基因的克隆及功能分析[J]. 中国水稻科学, 2014, 28(4): 343-350. |
| [5] | 刘希玲,邹华松,邹丽芳,陈功友 . 水稻白叶枯病菌hrcU基因缺失突变体构建及功能研究[J]. 中国水稻科学, 2010, 24(4): 348-352 . |
| [6] | 耿锐梅,傅扬,张文明,张建萍,余柳青,. 麦根腐平脐蠕孢和薏苡平脐蠕孢防治稻田稗草的生物活性和安全性[J]. 中国水稻科学, 2008, 22(3): 307-312 . |
| [7] | 万树青,刘祥发, 冯国忠,潘大建. 几种野生稻甲醇提取物对害虫的行为干扰及对酯酶同工酶的影响[J]. 中国水稻科学, 2006, 20(3): 340-342 . |
| [8] | 段桂芳,张建萍,周勇军,余柳青,袁勤生,. 禾长蠕孢菌及其代谢产物Ophiobolin A防治水稻纹枯病[J]. 中国水稻科学, 2006, 20(3): 337-339 . |
| [9] | 李湘民, 胡白石, 许志刚, MEW T. W.. 拮抗细菌Bacillus subtilis B5423-R 抑制水稻纹枯病的阈值群体数量[J]. 中国水稻科学, 2003, 17(4): 360-364 . |
| [10] | 郑爱萍, 李 平 , 王世全, 王玲霞, 马炳田. 水稻纹枯病菌拮抗菌B34分离鉴定及杀菌蛋白的获得[J]. 中国水稻科学, 2002, 16(4): 356-360 . |
| [11] | 唐家斌, 马炳田, 王玲霞, 李 平, 郑爱萍, 陈 红. 用木霉、类木霉对水稻纹枯病进行生物防治的研究[J]. 中国水稻科学, 2002, 16(1): 63-66 . |
| [12] | 谢关林. 中国长江三角洲地区及日本水稻种子细菌多样性研究[J]. 中国水稻科学, 2000, 14(4): 233-236 . |
| [13] | 赵新华,陈卫良,李德葆. 白叶枯病菌拮抗菌筛选及水稻叶围微生物互作研究初报[J]. 中国水稻科学, 2000, 14(3): 161-164 . |
| [14] | 陈志谊,许志刚,高泰东,倪寿坤,严大富,陆 凡,刘永锋. 水稻纹枯病拮抗细菌的评价与利用[J]. 中国水稻科学, 2000, 14(2): 98-102 . |
| [15] | 陈俊炜 ,张哲涛 ,蒋金炜 ,王世明 ,郭玉杰 ,王念英 . 昆虫病原线虫防治麦田越冬稻象甲的室内研究[J]. 中国水稻科学, 1996, 10(2): 125-128 . |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||