
中国水稻科学 ›› 2020, Vol. 34 ›› Issue (6): 586-594.DOI: 10.16819/j.1001-7216.2020.0101
• • 上一篇
张珏锋1, 张琴2, 李芳1, 钟海英1, 陈建明1,*( )
)
收稿日期:2020-01-06
修回日期:2020-04-20
出版日期:2020-11-10
发布日期:2020-11-10
通讯作者:
陈建明
基金资助:
Juefeng ZHANG1, Qin ZHANG2, Fang LI1, Haiying ZHONG1, Jianming CHEN1,*( )
)
Received:2020-01-06
Revised:2020-04-20
Online:2020-11-10
Published:2020-11-10
Contact:
Jianming CHEN
摘要: 目的 明确氯虫苯甲酰胺处理下二化螟中肠细菌类微生物多样性的变化。方法 综合运用宏基因组测序以及传统微生物分离纯化的方法分析不同浓度氯虫苯甲酰胺处理的二化螟种群中肠微生物的多样性。结果 不同浓度(100、200、400µg/mL)氯虫苯甲酰胺处理后二化螟中肠细菌类微生物丰度及多样性降低,处理种群中肠细菌类微生物的OTU数值、特有的OTU数值均低于对照;摩根氏菌属(Morganella)、普罗威登斯菌属(Providencia)以及变形菌属(Proteus)在处理种群中的比例明显升高;同时培养结果显示氯虫苯甲酰胺处理种群获得的菌株除肠杆菌属(Enterobacter)外,还存在超压莱略特氏菌(Lelliottianimipressuralis)、非脱羧勒菌(Leclerciaadecarboxylata)、成团泛菌(Pantoeaagglomerans)等其他菌属。COG数据库对比与KEGG分析显示,对照与处理二化螟种群的各功能分类基因数量及相对丰度无明显差异。结论 解析了氯虫苯甲酰胺胁迫下二化螟中肠细菌类微生物的多样性。这些结果为进一步研究中肠细菌在二化螟对氯虫苯甲酰胺的抗药性奠定基础。
中图分类号:
张珏锋, 张琴, 李芳, 钟海英, 陈建明. 氯虫苯甲酰胺胁迫下二化螟中肠细菌类微生物的多样性[J]. 中国水稻科学, 2020, 34(6): 586-594.
Juefeng ZHANG, Qin ZHANG, Fang LI, Haiying ZHONG, Jianming CHEN. Diversity of Midgut Microbial Community of ChilosuppressalisExposed to Chlorobenzamide[J]. Chinese Journal OF Rice Science, 2020, 34(6): 586-594.
| 样本 Sample | OUT (97%) | Ace指数 Ace index | Chao指数 Chao index | 测序深度指数 Sequencing depth | 香农指数 Shannon index | 辛普森指数 Simpson index |
|---|---|---|---|---|---|---|
| 100_24h | 333 | 442(407,494) | 405(377,452) | 0.99 | 2.19(2.18, 2.21) | 0.20(0.20, 0.20) |
| 100_48h | 432 | 758(693,838) | 640(571,742) | 0.99 | 2.39(2.37, 2.40) | 0.17(0.17, 0.17) |
| 100_72h | 580 | 718(680,771) | 745(690,827) | 0.99 | 2.98(2.96, 2.99) | 0.13(0.13, 0.13) |
| 200_24h | 412 | 512(481,557) | 500(467,553) | 0.99 | 2.02(2.00, 2.04) | 0.32(0.32, 0.32) |
| 200_48h | 357 | 415(394,448) | 407(385,447) | 0.99 | 2.49(2.47, 2.50) | 0.16(0.16, 0.16) |
| 200_72h | 639 | 742(713,782) | 730(698,778) | 0.99 | 3.02(3.01, 3.04) | 0.13(0.12, 0.13) |
| 400_24h | 474 | 578(547,623) | 569(535,623) | 0.99 | 2.70(2.68, 2.71) | 0.12(0.12, 0.12) |
| 400_48h | 751 | 901(863,952) | 893(849,956) | 0.99 | 3.31(3.29, 3.33) | 0.09(0.09, 0.09) |
| 400_72h | 321 | 539(473,634) | 557(473,690) | 0.99 | 2.22(2.20, 2.23) | 0.16(0.15, 0.16) |
| CK_24h | 1003 | 1127(1096,1169) | 1123(1085,1177) | 0.99 | 3.91(3.89, 3.93) | 0.07(0.07, 0.07) |
| CK_48h | 718 | 782(763,811) | 780(757,817) | 0.99 | 3.38(3.36, 3.40) | 0.08(0.09, 0.09) |
| CK_72h | 650 | 791(754,842) | 769(730,827) | 0.99 | 3.38(3.36, 3.39) | 0.08(0.08, 0.08) |
表1 不同处理二化螟试虫中肠细菌群落多样性指数
Table 1 Bacterial community richness, diversity indices of midgut in Chilosuppressalis under different treatments.
| 样本 Sample | OUT (97%) | Ace指数 Ace index | Chao指数 Chao index | 测序深度指数 Sequencing depth | 香农指数 Shannon index | 辛普森指数 Simpson index |
|---|---|---|---|---|---|---|
| 100_24h | 333 | 442(407,494) | 405(377,452) | 0.99 | 2.19(2.18, 2.21) | 0.20(0.20, 0.20) |
| 100_48h | 432 | 758(693,838) | 640(571,742) | 0.99 | 2.39(2.37, 2.40) | 0.17(0.17, 0.17) |
| 100_72h | 580 | 718(680,771) | 745(690,827) | 0.99 | 2.98(2.96, 2.99) | 0.13(0.13, 0.13) |
| 200_24h | 412 | 512(481,557) | 500(467,553) | 0.99 | 2.02(2.00, 2.04) | 0.32(0.32, 0.32) |
| 200_48h | 357 | 415(394,448) | 407(385,447) | 0.99 | 2.49(2.47, 2.50) | 0.16(0.16, 0.16) |
| 200_72h | 639 | 742(713,782) | 730(698,778) | 0.99 | 3.02(3.01, 3.04) | 0.13(0.12, 0.13) |
| 400_24h | 474 | 578(547,623) | 569(535,623) | 0.99 | 2.70(2.68, 2.71) | 0.12(0.12, 0.12) |
| 400_48h | 751 | 901(863,952) | 893(849,956) | 0.99 | 3.31(3.29, 3.33) | 0.09(0.09, 0.09) |
| 400_72h | 321 | 539(473,634) | 557(473,690) | 0.99 | 2.22(2.20, 2.23) | 0.16(0.15, 0.16) |
| CK_24h | 1003 | 1127(1096,1169) | 1123(1085,1177) | 0.99 | 3.91(3.89, 3.93) | 0.07(0.07, 0.07) |
| CK_48h | 718 | 782(763,811) | 780(757,817) | 0.99 | 3.38(3.36, 3.40) | 0.08(0.09, 0.09) |
| CK_72h | 650 | 791(754,842) | 769(730,827) | 0.99 | 3.38(3.36, 3.39) | 0.08(0.08, 0.08) |
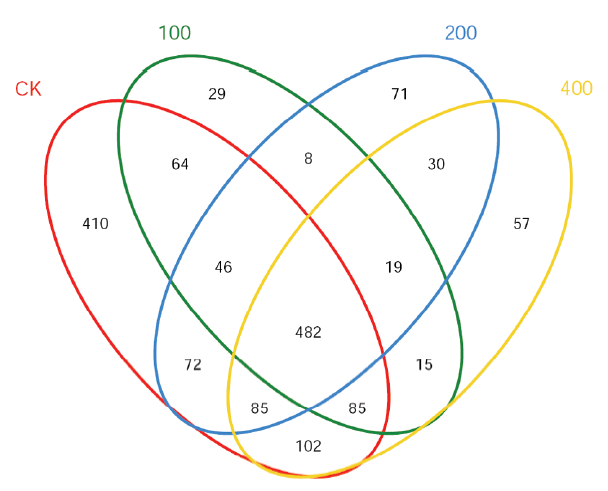
图1 不同处理二化螟试虫中肠细菌类微生物OUT韦恩图分析 图中红色为对照处理二化螟种群中肠细菌类微生物群落OUTs,绿色为100μg/mL氯虫苯甲酰胺处理二化螟种群中肠细菌类微生物群落OUTs,蓝色为200μg/mL氯虫苯甲酰胺处理二化螟种群中肠细菌类微生物群落OUTs,黄色为400μg/mL氯虫苯甲酰胺处理二化螟种群中肠细菌类微生物群落OUTs。
Fig. 1. Venn analysis of the number and community of intestinal bacteria of Chilosuppressalis under different treatments. The red circle is the outs of the intestinal bacterial community in the control population; the green circle, the outs of the intestinal bacterial community in the 100µg/mL chlorobenzamide treated population, the blue circle, the outs of the intestinal bacterial community in the 200µg/mL chlorobenzamide treated population, the yelloow, the outs of the intestinal bacterial community in the 400µg/mL chlorobenzamide treated population.
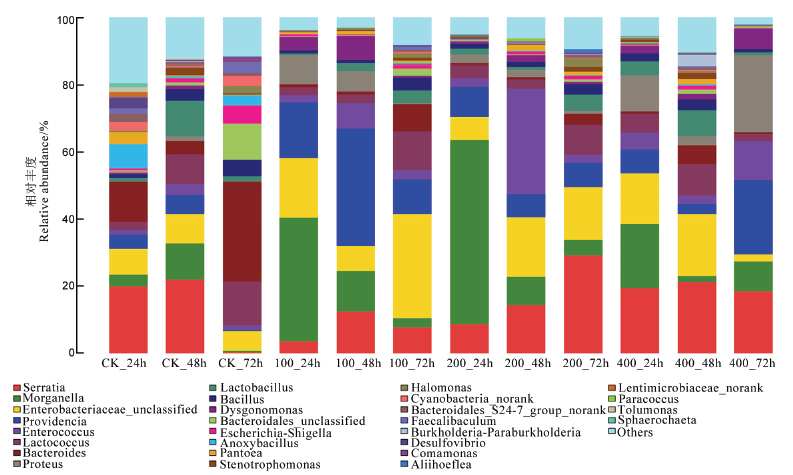
图2 基于属水平不同处理二化螟体内中肠细菌群落结构 Serratia–沙雷氏菌;Morganella–摩根氏菌;Enterobacteriaceae_unclassified–肠杆菌科(未确定);Providencia–普罗维登斯菌属;Enterococcus–肠球菌属;Lactococcus–乳球菌属;Bacteroides–拟杆菌属;Proteus–变形杆菌属;Lactobacillus–乳酸菌属;Bacillus–芽孢杆菌属;Dysgonomonas–营发酵单胞菌属;Bacteroidales_unclassified–拟杆菌目(未确定);Escherichia-Shigella–大肠杆菌属-志贺氏菌属;Anoxybacillus–无氧芽胞杆菌属;Pantoea–泛菌属;Stenotrophomonas–寡养单胞菌属;Halomonas–嗜盐单胞菌属;Cyanobacteria_norank–蓝细菌(未分类);Bacteroidales_S24-7_group_norank–拟杆菌目S24-7(未分类);Faecalibaculum–粪杆菌属;Burkholderia-Paraburkholderia–伯克霍尔德菌-拟伯克氏菌属;Desulfovibrio–脱磷孤菌属;Comamonas–丛毛单胞菌属;Aliihoeflea–砷氧化菌;Paracoccus–副球菌属;Tolumonas–甲苯单胞菌属;Sphaerochaeta–螺旋菌属;Others–其他。
Fig. 2. Genera of intestinal bacteria found in different treated Chilosuppressalis population.
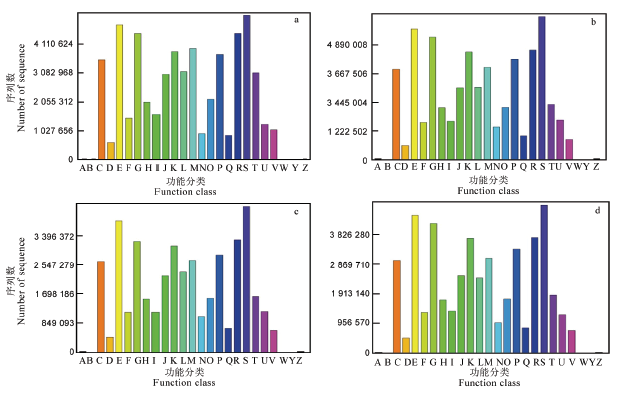
图3 不同处理二化螟试虫COG功能预测 A–RNA加工与修饰;B–染色质结构与动力学;C–能量产生与转化;D–细胞周期控制、细胞分裂、染色体分区;E–氨基酸运输和代谢;F–核苷酸运输和代谢;G–碳水化合物运输和代谢;H–辅酶运输和代谢;I–脂质运输和代谢;J–翻译、核糖体结构和生物起源;K–转录;L–复制、重组和修复;M–细胞壁/膜/信封生源论;N–细胞活性;O–翻译后修饰、蛋白质转换、分子伴侣;P–无机离子运输和代谢;Q–次级代谢产物生物合成、运输和分解代谢;R–通用功能预测;S–未知功能;T–信号转导机制;U–胞内运输、分泌和膜泡运输;V–防御机制;W–细胞外结构;Y–核结构;Z–细胞骨架。图中a为CK_24h,b为100_24h,c为200_24h,d为400_24h。
Fig. 3. COG classificationof intestinal bacteria of Chilosuppressalis in different treatments. A, RNA processing and modification; B, Chromatin structure and dynamics; C, Energy production and conversion; D, Cell cycle control, cell division, chromosome partitioning; E, Amino acid transport and metabolism; F, Nucleotide transport and metabolism;G, Carbohydrate transport and metabolism; H, Coenzyme transport and metabolism; I, Lipid transport and metabolism; J, Translation, ribosomal structure and biogenesis; K, Transcription; L, Replication, recombination and repair; M, Cell wall/membrane/envelope biogenesis; N, Cell motility; O, Posttranslational modification, protein turnover, chaperones; P, Inorganic ion transport and metabolism; Q, Secondary metabolites biosynthesis, transport and catabolism; R, General function prediction only; S, Function unknown; T, Signal transduction mechanisms; U, Intracellular trafficking, secretion, and vesicular transport; V, Defense mechanisms; W, Extracellular structures; Y, Nuclear structure; Z, Cytoskeleton. In the figure, a is CK_24h,b, 100_24h,c, 200_24h,d is 400_24h.
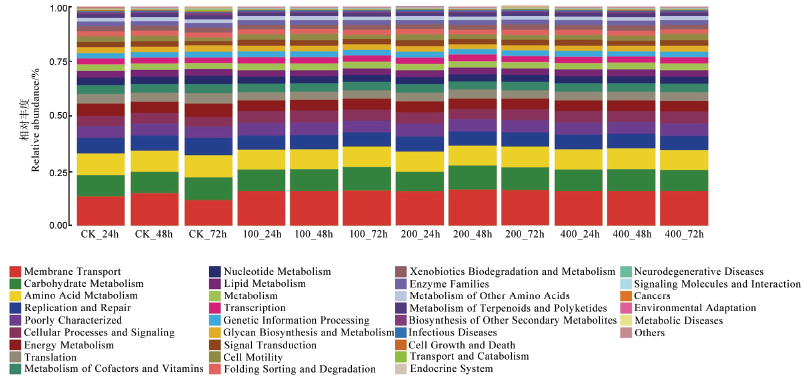
图4 基于宏基因组测序KEGG功能预测 Membrane transport–膜运输,Carbohydrate metabolism–水化合物代谢,Amino acid metabolism–氨基酸代谢,Replication and repair–复制和修复,Poorly characterized–缺乏特征,Cellular processes and signaling–细胞过程和信号,Energy metabolism–能量代谢,Translation–转译,Metabolism of cofactors and vitamins–辅酶因子和维生素代谢,Nucleotide metabolism–核苷酸代谢,Lipid metabolism–脂质代谢,Metabolism–代谢,Transcription–转录,Genetic information processing–遗传信息处理,Glycan biosynthesis and metabolism–多糖合成和代谢,Signal transduction–信号转导,Cell motility–细胞运动性,Folding, sorting and degradation–折叠,排序和退化,Xenobiotics biodegradation and metabolism–外源性生物降解与代谢,Enzyme families–酶家族,Metabolism of other amino acids–其他氨基酸代谢,Metabolism of terpenoids and polyketides–萜类和多酮类化合物代谢,Biosynthesis of other secondary metabolites–其他次生代谢物的生物合成,Infectious diseases–传染性疾病,Cell growth and death–细胞生长与死亡,Transport and catabolism–运输和分解代谢,Endocrine system–内分泌系统,Neurodegenerative diseases–神经退行性疾病,Signaling molecules and interaction–信号分子和相互作用,Cancers–癌症,environmental adaptation–环境适应,Metabolic diseases–代谢疾病,Others–其他。
Fig.4. KEGG function prediction based on macrogenomic sequencing.
| 分类 Classification | 对照CK | 100µg/mL | 200µg/mL | 400µg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | ||||
| 肠杆菌属 Enterobacter | 霍氏肠杆菌 Enterobacter hormaechei | 4 | 霍氏肠杆菌 Enterobacter hormaechei | 3 | |||||||
| 阿氏肠杆菌YT Enterobacter asburiae strain YT | 1 | 阿氏肠杆菌YT Enterobacter asburiaestrain YT | 2 | ||||||||
| 肠杆菌属 CZBSY4Enterobacter sp. CZBSY4 | 4 | 肠杆菌属 CZBSY4 Enterobacter sp. CZBSY4 | 1 | ||||||||
| 肠杆菌属 09-M1 Enterobacter sp. 09-M1 | 2 | ||||||||||
| 肠杆菌属 XM Enterobacter sp. strain XM | 3 | ||||||||||
| 阴沟肠杆菌 Enterobacter cloacaether | 1 | ||||||||||
| 路氏肠杆菌 Enterobacter ludwigii | 2 | ||||||||||
| 肠杆菌属 S4(2015) Enterobacter sp. S4(2015) | 4 | 肠杆菌属 S4(2015) Enterobacter sp. S4(2015) | 1 | ||||||||
| 产气肠杆菌 | 2 | 产气肠杆菌 | 1 | 产气肠杆菌 | 1 | ||||||
| Enterobacter roggenkampii Enterobacter aerogenes | 1 | Enterobacter roggenkampii Enterobacter aerogenes | 3 | Enterobacter roggenkampii Enterobacter aerogenes | 1 | ||||||
| 森肠杆菌 Enterobacter mori | 1 | 森肠杆菌 Enterobacter mori | 2 | 森肠杆菌 Enterobacter mori | 1 | ||||||
| 河生肠杆菌 Enterobacter amnigenus | 3 | 河生肠杆菌 Enterobacter amnigenus | 4 | ||||||||
| 神户肠杆菌 Enterobacter kobei | 1 | 神户肠杆菌 Enterobacter kobei | 1 | ||||||||
| 阿氏肠杆菌 Enterobacter asburiae | 3 | ||||||||||
| 其他 Others | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 2 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 1 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 2 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 5 | |||
| 成团泛菌 Pantoeaagglomerans | 1 | 成团泛菌 Pantoeaagglomerans | 3 | 成团泛菌 Pantoeaagglomerans | 2 | ||||||
| 泛菌属DAP16 Pantoea sp. DAP16 | 1 | 泛菌属DAP16 Pantoea sp. DAP16 | 2 | 泛菌属DAP16 Pantoea sp. DAP16 | 2 | ||||||
| 克雷伯氏菌属 Klebsiella sp. | 1 | 克雷伯氏菌属 Klebsiella sp. | 1 | ||||||||
| 超压莱略特氏菌 Lelliottianimipressuralis | 1 | 超压莱略特氏菌 Lelliottianimipressuralis | 1 | ||||||||
| ɤ-变形菌门细菌 Gammaproteobacteria bacterium strain | 3 | ɤ-变形菌门细菌 Gammaproteobacteria bacterium strain | 1 | ||||||||
| 不可培养细菌 Uncultured bacterium | 3 | 不可培养细菌 Uncultured bacterium | 3 | 不可培养细菌 Uncultured bacterium | 1 | ||||||
表2 二化螟中肠细菌类微生物离体培养测序结果
Table 2 Isolation and identification of bacteria from the midgut ofChilosuppressalis.
| 分类 Classification | 对照CK | 100µg/mL | 200µg/mL | 400µg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | 菌株名称 Strain | 数量 Quantity | ||||
| 肠杆菌属 Enterobacter | 霍氏肠杆菌 Enterobacter hormaechei | 4 | 霍氏肠杆菌 Enterobacter hormaechei | 3 | |||||||
| 阿氏肠杆菌YT Enterobacter asburiae strain YT | 1 | 阿氏肠杆菌YT Enterobacter asburiaestrain YT | 2 | ||||||||
| 肠杆菌属 CZBSY4Enterobacter sp. CZBSY4 | 4 | 肠杆菌属 CZBSY4 Enterobacter sp. CZBSY4 | 1 | ||||||||
| 肠杆菌属 09-M1 Enterobacter sp. 09-M1 | 2 | ||||||||||
| 肠杆菌属 XM Enterobacter sp. strain XM | 3 | ||||||||||
| 阴沟肠杆菌 Enterobacter cloacaether | 1 | ||||||||||
| 路氏肠杆菌 Enterobacter ludwigii | 2 | ||||||||||
| 肠杆菌属 S4(2015) Enterobacter sp. S4(2015) | 4 | 肠杆菌属 S4(2015) Enterobacter sp. S4(2015) | 1 | ||||||||
| 产气肠杆菌 | 2 | 产气肠杆菌 | 1 | 产气肠杆菌 | 1 | ||||||
| Enterobacter roggenkampii Enterobacter aerogenes | 1 | Enterobacter roggenkampii Enterobacter aerogenes | 3 | Enterobacter roggenkampii Enterobacter aerogenes | 1 | ||||||
| 森肠杆菌 Enterobacter mori | 1 | 森肠杆菌 Enterobacter mori | 2 | 森肠杆菌 Enterobacter mori | 1 | ||||||
| 河生肠杆菌 Enterobacter amnigenus | 3 | 河生肠杆菌 Enterobacter amnigenus | 4 | ||||||||
| 神户肠杆菌 Enterobacter kobei | 1 | 神户肠杆菌 Enterobacter kobei | 1 | ||||||||
| 阿氏肠杆菌 Enterobacter asburiae | 3 | ||||||||||
| 其他 Others | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 2 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 1 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 2 | 非脱羧埃希氏菌 Leclerciaadecarboxylata | 5 | |||
| 成团泛菌 Pantoeaagglomerans | 1 | 成团泛菌 Pantoeaagglomerans | 3 | 成团泛菌 Pantoeaagglomerans | 2 | ||||||
| 泛菌属DAP16 Pantoea sp. DAP16 | 1 | 泛菌属DAP16 Pantoea sp. DAP16 | 2 | 泛菌属DAP16 Pantoea sp. DAP16 | 2 | ||||||
| 克雷伯氏菌属 Klebsiella sp. | 1 | 克雷伯氏菌属 Klebsiella sp. | 1 | ||||||||
| 超压莱略特氏菌 Lelliottianimipressuralis | 1 | 超压莱略特氏菌 Lelliottianimipressuralis | 1 | ||||||||
| ɤ-变形菌门细菌 Gammaproteobacteria bacterium strain | 3 | ɤ-变形菌门细菌 Gammaproteobacteria bacterium strain | 1 | ||||||||
| 不可培养细菌 Uncultured bacterium | 3 | 不可培养细菌 Uncultured bacterium | 3 | 不可培养细菌 Uncultured bacterium | 1 | ||||||
| [1] | Sharon G, Segal D, Ringo JM.Commensal bacteria play a role in mating preference of Drosophila melanogaster[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010,107(46):20051-20056. |
| [2] | Dillon R J, Vennard C T, Buckling A.Diversity of locust gut bacteria protects against pathogen invasion[J]. Ecology Letters, 2005, 8(12):1291-1298. |
| [3] | Roush R T, McKenzie J A. Ecological genetics of insecticide and acaricideresistance[J]. Annual Review of Entomology,1987, 32:361-380. |
| [4] | Whalon M E, Motasanchez D, Hollingworth R M.Global pesticide resistance in arthropods[M]. Cambridge, Mass:Wallingford, Oxfordshire,2008 |
| [5] | 张浩, 薛妍, 候艳飞. 肠道菌对苏云菌芽孢杆菌杀虫活性的研究[J]. 生物技术通报, 2012(7): 176-180. (in Chinese with English abstract) |
| Zhang H, Xue Y, Hou Y F.Effects of gut bacteria to the insecticidal activity of Bacillus thuringiensistoward Helicoverpaarmigera[J]. Biotechnology Bulletin, 2012(7): 176-180. | |
| [6] | Kikuchia Y, Hayatsuc M, Hosokawad T.Symbiont-mediated insecticide resistance[J]. Proceedingsof the National Academy of Sciences, 2012,109(22): 8612-8617. |
| [7] | 刘浩,张凡,黄艳红. 三种抗生素对德国小蠊肠道菌去除效果的研究[J].山东师范大学学报, 2012, 27(3): 115-117. |
| Liu H, Zhang F, Huang Y H.A Study of the removal of intestinal flora in BlattellaGermanica using three antibiotics[J].Journal of Shandong Normal University, 2012, 27(3): 115-117. (in Chinese with English abstract) | |
| [8] | 刘浩. 德国小蠊共生菌种群变化与抗药性的关系[D]. 济南: 山东师范大学, 2013. |
| Liu H.Study on the symbiotic bacteria population change and insecticide resistance of Blattellagermannica[D]. Jinan: Shandong Normal University, 2013. (in Chinese with English abstract) | |
| [9] | Xia X F, Zheng D D, You M S.DNA sequencing reveals the midgut microbiota of diamondback moth, Plutellaxylostella (L.) and a possible relationship with insecticide resistance[J]. PLoS ONE,2013, 8(7):e68852. |
| [10] | 夏晓峰. 小菜蛾中肠微生物多样性及其功能研究[D]. 福州: 福建农林大学, 2014. |
| Xia X F.Organizational diversity and functional characterization of microbiota in the midgut of diamondback moth, Plutellaxylostella L. [D]. Fuzhou: Fujian Agriculturaland Forestry University, 2014. (in Chinese with English abstract) | |
| [11] | 胡君, 陈文明, 张真真, 郑雪松, 靳建超, 苏建亚, 高聪芬, 沈晋良. 长江流域稻区二化螟抗药性监测. 中国水稻科学, 2010, 24(5): 509-515. |
| Hu J, Chen W M, Zhang Z Z, Zheng X S, Jin J C, Su J Y, Gao C F, Shen J L.Insecticide resistance monitoring of Chilosuppressalis in the drainage area of the Yangtze River, China[J].Chinese Journal of Rice Science,2010, 24(5): 509-515. (in Chinese with English abstract) | |
| [12] | 张扬, 王保菊, 韩平, 韩召军. 二化螟抗药性检测方法比较和抗药性监测. 南京农业大学学报, 2014, 37(6):37-43. |
| Zhang Y, Wang B J, Han P, Han Z J.Comparison of methods for testing insecticide resistance in Chilosuppressalis and the resistance monitored[J].Journal of Nanjing Agricultural University, 2014, 37(6):37-43. (in Chinese with English abstract) | |
| [13] | Yao R, Zhao DD, Zhang S, Zhou LQ, Wang X, Gao CF, Wu SF.Monitoring and mechanisms of insecticide resistance in Chilosuppressalis (Lepidoptera: Crambidae), with special reference to diamides[J]. Pest Management,2017, 73: 1169-1178. |
| [14] | He Y P, Zhang J F, Gao C F.Regression analysis of dynamics of insecticide resistance in field populations of Chilosuppressalis (Walker) (Lepidoptera: Pyralidae) during 2002-2011 in China[J]. Journal of Economic Entomology, 2013,106(4): 1832-1837. |
| [15] | He Y P, Zhang J F, Chen J M.Effect of synergists on susceptibility to chlorantraniliprole in field populations of rice stem borer (Chilosuppressalis) (Lepidoptera: Pyralidae)[J]. Journal of Economic Entomology, 2014, 107(2): 791-796. |
| [16] | Su J Y, Zhang Z Z, Gao C F.Geographic susceptibility of Chilosuppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China[J].Pest Management Science, 2014,70(6):989-995. |
| [17] | Lu Y H, Wang G R, Zhong L Q, Zhang F C, Bai Q,Zheng X S, Lu Z X.Resistance monitoring of Chilosuppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from east and central China[J]. Crop Protection, 2017, 100: 196-202. |
| [18] | Kang W J, Koo H N,Jeong D, Kim H. Functional and genetic characteristics of chlorantraniliprole resistance in the diamondback moth, Plutellaxylostella(Lepidoptera: Plutellidae)[J]. Entomological Research, 2017, 47(6):394-403. |
| [19] | Hu Z D, Feng X, Lin Q S, Chen H Y, Li Z Y, Yin F.Biochemical mechanism of chlorantraniliprole resistance in the diamondback moth, plutellaxylostellalinnaeus[J]. Journal of Integrative Agriculture, 2014, 11:2452-2459. |
| [20] | 张珏锋, 何月平, 陈建明. 不同抗性水平二化螟幼虫中肠细菌群落多样性分析[J]. 昆虫学报, 2013, 56(9): 1075-1082. |
| Zhang J F, He Y P, Chen J M.Diversity analysis of bacterial community in midguts of larvae of the striped stem borer,Chilosuppressalis(Lepidoptera:Crambidae), with different levels of resistance to insecticides ActaEntomologica Sinica, 2013, 56(9): 1075-1082. (in Chinese with English abstract) | |
| [21] | Gandhi GracyR, MalathiV M, JalaliS K,Thulasi A. Variation in larval gut bacteria between insecticide-resistant and -susceptible populations of Helicoverpaarmigera (Hübner)(Lepidoptera: Noctuidae)[J].Phytoparasitica, 2016, 44:477-490. |
| [22] | Huang S, Zhang H.The impact of environmental heterogeneity and life stage on the hindgut microbiota ofHolotrichiaparallela larvae (Coleoptera: Scarabaeidae)[J/OL]. PloSONE, 2013, 8(2):e57169. |
| [23] | Nyholm S V, Graf J.Knowing your friends: Invertebrate innate immunity fosters beneficial bacterial symbioses[J]. Nature Reviews Microbiology, 2012, 10(12): 815-827. |
| [24] | Round JL, Mazmanian SK.The gut microbiota shapes intestinal immune responses during health and disease[J]. Nature Reviews Immunology, 2009, 9(5): 313-323. |
| [25] | Robertson B K, Alexander M.Growth-linked and cometabolic biodegradation: Possible reason for occurrence or absence of accelerated pesticide biodegradation[J]. Pesticide Science,1994, 41(4):311-324. |
| [26] | Sonia Rodríguez-Cruz M, Jones J E, Bending G D. Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics[J]. Soil Biology and Biochemistry. 2006, 38(9):2910-2918. |
| [27] | Cheng D F, GuoZ J,Riegler M. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel)[J].Microbiome, 2017, 5:13. |
| [28] | 李冠楠. 氟胁迫对不同抗性家蚕肠道微生态环境的影响[D]. 重庆: 西南大学,2015. |
| Li G N.Effect of fluoride exposure on the intestinal microecology in different-resistance of silkworm, Bombyxmori L. [D]. Chongqing: Southwest University, 2015. (in Chinese with English abstract) | |
| [29] | 刘金萍. 高CO2浓度对棉铃虫适合度及肠道微生物的直接影响[D]. 武汉: 华中农业大学, 2017. |
| Liu J P.The direct effects of elevated CO2 on fitness and gut microbes of HelicoverpaArmigera[D]. Wuhan: Huazhong Agricultural University, 2017. (in Chinese with English abstract) | |
| [30] | Louca S, Parfrey L W, Doebeli M.Decoupling function and taxonomy in the global oceanmicrobiome[J]. Science, 2016, 353(6305): 1272-1277. |
| [31] | Nelson M B, Martiny A C, Martiny J B H. Global biogeography of microbial nitrogen-cycling traits in soil[J]. Proceedingsof the National Academy of Sciences, 2016, 113(29): 8033-8040. |
| [1] | 刘艳, 何林凤, 汪书超, 杨凤霞, 高聪芬, 吴顺凡. 二化螟对甲氧虫酰肼的抗性风险、交互抗性及亚致死效应研究[J]. 中国水稻科学, 2023, 37(4): 427-435. |
| [2] | 刘琴, 夏杨, 韩光杰, 李传明, 陆玉荣, 黄立鑫, 祁建杭, 徐健. 水稻二化螟病原线虫N-Yz1的分离鉴定及其感染特性[J]. 中国水稻科学, 2022, 36(6): 639-646. |
| [3] | 宋瑞雪, 鲁涵, 鲁艳辉, 郑许松, 吕仲贤. 取食香根草后水稻螟虫对杀虫剂敏感度变化[J]. 中国水稻科学, 2019, 33(3): 282-286. |
| [4] | 徐红星, 王国荣, 鲁艳辉, 杨亚军, 郑许松, 田俊策, 吕仲贤. 二化螟实时荧光定量PCR内参基因筛选和表达稳定性评价[J]. 中国水稻科学, 2019, 33(1): 75-84. |
| [5] | 赵丹丹, 周丽琪, 张帅, 姚蓉, 邱运霞, 高聪芬. 二化螟对双酰胺类杀虫剂的抗药性监测和交互抗性研究[J]. 中国水稻科学, 2017, 31(3): 307-314. |
| [6] | 钟海英, 张珏锋, 李芳, 陈建明. 二化螟水稻、茭白种群幼虫口器和触角及其感器扫描电镜观察[J]. 中国水稻科学, 2017, 31(2): 195-206. |
| [7] | 李晓欢1, 2, #,罗光华2, #,张志春2,刘宝生2,方继朝1, 2,*. 二化螟Ty3/gypsy反转座子的克隆与序列分析[J]. 中国水稻科学, 2014, 28(3): 314-321. |
| [8] | 胡阳1,郑永利2,曹国连1,傅强1,*. 利用半人工饲料大规模简便化饲养二化螟[J]. 中国水稻科学, 2013, 27(5): 535-538. |
| [9] | 罗光华1,张志春1,韩光杰1,韩召军2,方继朝1, *. 二化螟越冬种群特点及其对三唑磷靶标抗性突变频率分析[J]. 中国水稻科学, 2012, 26(4): 481-486. |
| [10] | 强承魁1,2,# ,于玲雅3,#,杜予州1,*,秦越华2,赵虎2,胡长效2. 快速冷耐受对水稻二化螟滞育幼虫的生理效应[J]. 中国水稻科学, 2012, 26(2): 251-254. |
| [11] | 郭震1,阮长春1,臧连生1,*,张帆2,*,靳锋云3 . 稻螟赤眼蜂rDNA特异引物设计及诊断引物在赤眼蜂分子鉴定中的应用[J]. 中国水稻科学, 2012, 26(1): 123-126. |
| [12] | 胡君,陈文明,张真真,郑雪松,靳建超,苏建亚,高聪芬,沈晋良, . 长江流域稻区二化螟抗药性监测 [J]. 中国水稻科学, 2010, 24(5): 509-515 . |
| [13] | 李冉, Sumera AFSHEEN,周国鑫,任 楠, 娄永根. 水稻二化螟诱导表达基因OsSABATH启动子的克隆与缺失体构建[J]. 中国水稻科学, 2008, 22(5): 454-458 . |
| [14] | 何月平,邵振润, 陈文明,梁桂梅,李永平,周威君,沈晋良,. 防治水稻二化螟的高毒农药替代药剂的室内筛选[J]. 中国水稻科学, 2008, 22(3): 313-320 . |
| [15] | 徐红星,吕仲贤,蒋学辉,俞晓平,陈建明,郑许松. 水稻植株在二化螟为害后的生理变化[J]. 中国水稻科学, 2007, 21(3): 316-318 . |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||