
中国水稻科学 ›› 2020, Vol. 34 ›› Issue (2): 115-124.DOI: 10.16819/j.1001-7216.2020.9122
王昌健, 陈龙, 代丽萍, 路雪丽, 贺金立, 杨龙, 胡江, 朱丽, 董国军, 张光恒, 高振宇, 任德勇, 陈光, 沈兰, 张强, 郭龙彪, 钱前*( ), 曾大力*(
), 曾大力*( )
)
收稿日期:2019-11-15
修回日期:2019-12-27
出版日期:2020-03-10
发布日期:2020-03-10
通讯作者:
钱前,曾大力
作者简介:#共同第一作者
基金资助:
Changjian WANG, Long CHEN, Liping DAI, Xueli LU, Jinli HE, Long YANG, Jiang HU, Li ZHU, Guojun DONG, Guangheng ZHANG, Zhenyu GAO, Deyong REN, Guang CHEN, Lan SHEN, Qiang ZHANG, Longbiao GUO, Qian QIAN*( ), Dali ZENG*(
), Dali ZENG*( )
)
Received:2019-11-15
Revised:2019-12-27
Online:2020-03-10
Published:2020-03-10
Contact:
Qian QIAN, Dali ZENG
About author:#These authors contributed equally to this work
摘要:
【目的】鉴定和克隆花器官发育相关基因,为进一步研究水稻花发育的分子机制奠定基础。【方法】在大田常规种植条件下比较了突变体dps2 (Defective pistil and stamens 2)和野生型春江06的主要农艺性状及花器官形态特征差异;扫描电镜及石蜡切片观察花药结构并用染色法观察花粉和胚囊的育性;利用图位克隆方法进行基因精细定位;qRT-PCR分析了花发育相关基因在野生型和突变体中的表达水平。【结果】dps2突变体抽穗期变长,不能正常扬花,雄蕊和雌蕊皱缩且花药和柱头数目增多;进一步研究发现,dps2突变体花药腔室塌陷,内无可见小孢子,即使部分花药形成腔室,花粉粒也无淀粉积累呈干瘪状。此外,突变体胚囊育性也受到影响;遗传分析表明该突变性状受一对隐性核基因控制,该基因位于第4染色体短臂上91.2 kb的区间内,区间内未见花器官发育相关基因的报道。qRT-PCR检测发现,水稻ABCDE模型中的B类、C类和E类基因的表达在突变体中显著升高。【结论】dps2突变体的雄蕊及雌蕊均发育异常,最终导致完全不育,推测DPS2可能在水稻第3轮雄蕊发育和第4轮雌蕊发育调控中发挥重要作用。
中图分类号:
王昌健, 陈龙, 代丽萍, 路雪丽, 贺金立, 杨龙, 胡江, 朱丽, 董国军, 张光恒, 高振宇, 任德勇, 陈光, 沈兰, 张强, 郭龙彪, 钱前, 曾大力. 水稻花器官发育基因DPS2的鉴定和精细定位[J]. 中国水稻科学, 2020, 34(2): 115-124.
Changjian WANG, Long CHEN, Liping DAI, Xueli LU, Jinli HE, Long YANG, Jiang HU, Li ZHU, Guojun DONG, Guangheng ZHANG, Zhenyu GAO, Deyong REN, Guang CHEN, Lan SHEN, Qiang ZHANG, Longbiao GUO, Qian QIAN, Dali ZENG. Identification and Fine Mapping of Defective Pistil and Stamens 2 in Rice[J]. Chinese Journal OF Rice Science, 2020, 34(2): 115-124.
| 引物名称 | 前引物 | 后引物 |
|---|---|---|
| Primer name | Forward primer(5'-3') | Reverse primer(5'-3') |
| BY-4 | GGAGGTGACTACGAAGGCATT | GAAGCTTATCCTTTTCACTTTCG |
| BY19 | GAACTCCAAGTCCACCCTGAT | GATAAATTCTGTTGGTGGGAGATTG |
| BY5 | GTTCACCAATAATCACCACAAGAGC | TAGCACCCCAGCAAGGACTCG |
| BY6-2 | CTCGGCTCTCTTTGCTCGG | TGCCGACGAGGTCACCGCC |
| BY7-3 | TATTTAGCCCTGGTTGAATGGT | TACCTAAGCTAAGTGGAAAGA |
| BY20 | GACCAGAGGTGGACCAAGGA | GTGAATGGAAAGTGTGTGAAAGATC |
| BY10-1 | GACTATATAGATATGTCCTCGGAAG | TTACCTATAACGAACCAAAGGC |
| BY13 | GGAAACAATATACTATGGTGTGGAT | CGTGGGTGGAGTTTAGAATG |
表1 部分实验引物
Table 1 Part of primers used in the research.
| 引物名称 | 前引物 | 后引物 |
|---|---|---|
| Primer name | Forward primer(5'-3') | Reverse primer(5'-3') |
| BY-4 | GGAGGTGACTACGAAGGCATT | GAAGCTTATCCTTTTCACTTTCG |
| BY19 | GAACTCCAAGTCCACCCTGAT | GATAAATTCTGTTGGTGGGAGATTG |
| BY5 | GTTCACCAATAATCACCACAAGAGC | TAGCACCCCAGCAAGGACTCG |
| BY6-2 | CTCGGCTCTCTTTGCTCGG | TGCCGACGAGGTCACCGCC |
| BY7-3 | TATTTAGCCCTGGTTGAATGGT | TACCTAAGCTAAGTGGAAAGA |
| BY20 | GACCAGAGGTGGACCAAGGA | GTGAATGGAAAGTGTGTGAAAGATC |
| BY10-1 | GACTATATAGATATGTCCTCGGAAG | TTACCTATAACGAACCAAAGGC |
| BY13 | GGAAACAATATACTATGGTGTGGAT | CGTGGGTGGAGTTTAGAATG |
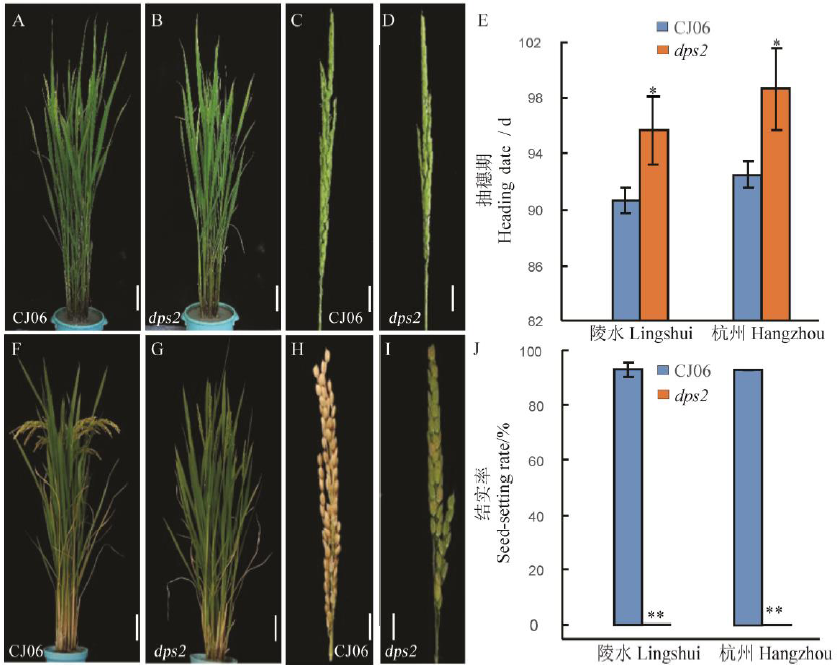
图1 突变体dps2及其突变体在抽穗期和成熟期的表型比较 A, B-抽穗期植株; C, D-抽穗期穗部表型; E-在海南陵水和浙江杭州的抽穗期; F, G-成熟期植株; H, I-成熟期穗部表型, J-在海南陵水和浙江杭州的结实率。误差线表示3次独立实验的标准差。*和**分别表示野生型与突变体间差异达0.05和0.01显著水平(t检验)。在A, B, F, G图中,标尺为10 cm; C, D, H, I标尺为2 cm。CJ06-春江06。
Fig. 1. Phenotypic comparison of dps2 mutant and wild type CJ06 at heading and maturity stage. A and B, Plants of Chunjiang 06(CJ06) and dps2 mutant at heading date; C and D, Panicle of CJ06 and dps2 at heading date; E, Heading date of CJ06 and dps2 in Lingshui, Hainan and Hangzhou, Zhejiang; F and G, Plants of CJ06 and dps2 at maturity stage; H and I, Panicle of CJ06 and dps2 at maturity stage; J, Seed-setting rate of CJ06 and dps2 in Lingshui and Hangzhou. Mean±SD(n=3). * and ** The difference between the wild type and mutant is significant at 0.05 and 0.01 level, respectively according to Student’s t test. In A, B, F, G, Bar=10 cm; In C, D, H, I, Bar=2 cm.
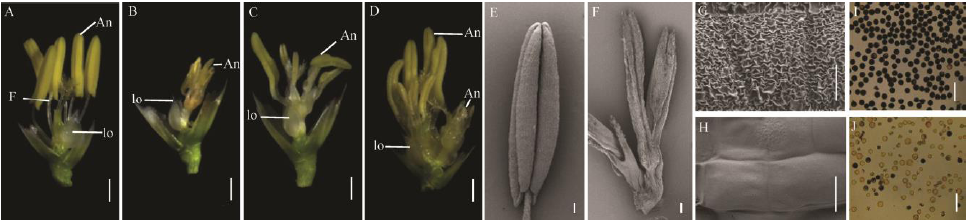
图2 野生型春江06与突变体dps2的雄蕊表型 A-野生型去除内外稃的颖花; B~D-突变体dps2去除内外稃的颖花; E-野生型花药; F-dps2花药; G-野生型花药外壁; H-dps2花药外壁; I-野生型花粉镜检; J-dps2花粉镜检。lo-浆片; An-花药; F-花丝。A~D图中标尺为1 mm, E, F标尺为100 μm, G, H标尺为10 μm, I, J标尺为50 μm。
Fig. 2. Stamens phenotypic observation of wild type Chunjiang 06(CJ06) and dps2 mutant. A, Glumous flower of CJ06; B to D, Glumous flower of dps2 mutant; E, F, Anthers of the wild type and dps2; G, H, The outmost surface on epidermis anthers of the wild type and dps2; I, J, Pollens of CJ06 and dps2 mutant. Lo, Lodicule; An, Anther; F, Filament. From A to D, Bar=1 mm; In E, F, Bar=25 μm; In G, H, Bar=10 μm; In I, J, Bar=50 μm.
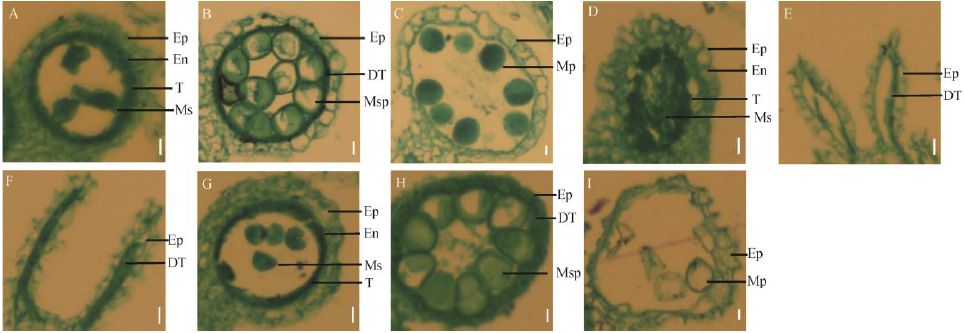
图3 野生型春江06与突变体dps2不同发育时期花药石蜡切片观察 A到C为野生型; D到I为突变体dps2。A, D和G-花药发育第9时期花药横切面; B, E和H-第10时期花药横切面; C, F和I-第12时期花药横切面。Ep-表皮层; En-内皮层; T-绒毡层; DT-退化绒毡层; Ms-小孢子母细胞; Msp-小孢子; Mp-成熟花粉。标尺为10 μm。
Fig. 3. Transverse section of anthers of dps2 mutant and wild type Chunjiang 06(CJ06) at different stages. A to C, CJ06; D to I, dps2 mutant; A, D, G, Cross-section of anther at the stage 9; B, E, H, Cross-section of anther at the stage 10; C, F, I, Cross-section of anther at the stage 12; Ep, Epidermis; En, Endothecium; T, Tapetum; DT, Degeneration tapetum; Ms, Microsporocyte; Msp, Microspore; Mp, Mature pollen. Bar=10 μm.
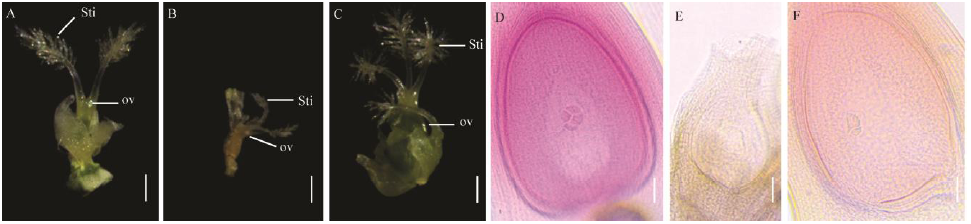
图4 野生型春江06与突变体dps2雌蕊表型观察 A-野生型春江06的雌蕊形态; B, C-突变体dps2的雌蕊形态; D-春江06胚囊镜检; E, F-突变体dps2胚囊镜检。Sti-柱头; ov-子房。在A~C图中,标尺为1 mm;在D~F图中,标尺为50 μm。
Fig. 4. Pistils phenotypic observation of wild type Chunjiang 06(CJ06) and dps2 mutant. A, Pistils of wild type; B, C, Pistils of dps2 mutant; D, Embryo sac of wild type; E, F, Embryo sac of dps2 mutant. Sti, Stigma; ov, Ovary. Scale bars: 1 mm in A, B and C, 50 μm in D to F.
| 杂交组合 Hybridized combination | F1 | F2 | χ2(3: 1) | χ2(0.05) | ||||
|---|---|---|---|---|---|---|---|---|
| 正常表型 Wild-type phenotype | 突变表型 dps2 phenotype | 正常表型 Wild-type phenotype | 突变表型 dps2 phenotype | |||||
| DPS2dps2/日本晴 DPS2dps2/Nipponbare | 7 | 0 | 236 | 79 | 0.001 | 3.84 | ||
| 日本晴/DPS2dps2 Nipponbare/DPS2dps2 | 4 | 0 | 133 | 43 | 0.030 | 3.84 | ||
| DPS2dps2/9311 | 5 | 0 | 160 | 42 | 1.908 | 3.84 | ||
| 9311/ DPS2dps2 | 4 | 0 | 127 | 35 | 0.996 | 3.84 | ||
表2 dps2突变位点的遗传分析
Table 2 Genetic analysis of the dps2 locus
| 杂交组合 Hybridized combination | F1 | F2 | χ2(3: 1) | χ2(0.05) | ||||
|---|---|---|---|---|---|---|---|---|
| 正常表型 Wild-type phenotype | 突变表型 dps2 phenotype | 正常表型 Wild-type phenotype | 突变表型 dps2 phenotype | |||||
| DPS2dps2/日本晴 DPS2dps2/Nipponbare | 7 | 0 | 236 | 79 | 0.001 | 3.84 | ||
| 日本晴/DPS2dps2 Nipponbare/DPS2dps2 | 4 | 0 | 133 | 43 | 0.030 | 3.84 | ||
| DPS2dps2/9311 | 5 | 0 | 160 | 42 | 1.908 | 3.84 | ||
| 9311/ DPS2dps2 | 4 | 0 | 127 | 35 | 0.996 | 3.84 | ||
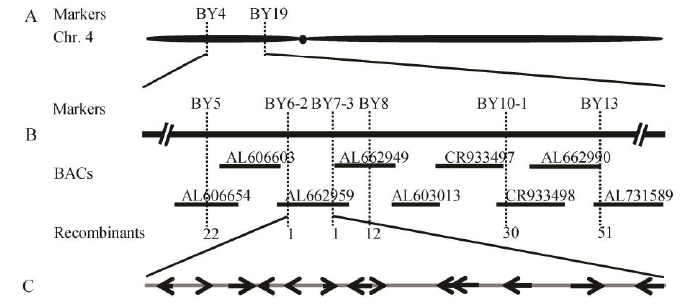
图5 DPS2在第4染色体上的定位 A-突变基因的初步定位; B-突变基因的精细定位; C-标记BY6-2和BY7-3区间内开发阅读框。
Fig. 5. Location of DPS2 on rice chromosome 4. A, Preliminary localization of DPS2 gene; B, Fine mapping of DPS2 gene; C, ORFs between markers BY6-2 and BY7-3.
| 登录号 | 位置 | 功能注释 |
|---|---|---|
| Gene accession | Location/bp | Functional annotation |
| LOC_Os04g08290 | 4 427 683-4 424 830 | ZOS4-04-C2H2锌指蛋白 ZOS4-04-C2H2 zinc finger protein |
| LOC_Os04g08300 | 4 433 624-4 433 839 | 反转录转座子蛋白,Ty3-gypsy亚类 Retrotransposon protein, putative, Ty3-gypsy subclass |
| LOC_Os04g08310 | 4 436 856-4 441 657 | 表达蛋白 Expressed protein |
| LOC_Os04g08320 | 4 444 004-4 441 881 | 假定蛋白YIF1B YIF1B, putative |
| LOC_Os04g08330 | 4 446 960-4 446 649 | 表达蛋白 Expressed protein |
| LOC_Os04g08340 | 4 452 696-4 455 350 | OsSigP3-假定的Ⅰ型信号肽酶同系物;采用假定的Ser/Lys催化二元体 OsSigP3-putative Type I signal peptidase homologue, employs a putative Ser/Lys catalytic dyad |
| LOC_Os04g08350 | 4 461 845-4 457 458 | 半胱氨酸合酶,叶绿体/染色质前体 Cysteine synthase, chloroplast/chromoplast precursor |
| LOC_Os04g08360 | 4 463 046-4 463 552 | 表达蛋白 Expressed protein |
| LOC_Os04g08370 | 4 473 512-4 470 642 | 富含亮氨酸的重复家族蛋白 Leucine rich repeat family protein |
| LOC_Os04g08390 | 4 490 101-4 484 997 | 富含亮氨酸的重复家族蛋白 Leucine rich repeat family protein |
| LOC_Os04g08400 | 4 497 789-4 503 741 | 反转录转座子蛋白 Retrotransposon protein |
| LOC_Os04g08410 | 4 513 883-4 508 804 | 含有ELM2结构域蛋白 ELM2 domain containing protein |
表3 第4染色体候选区间内注释的12个基因
Table 3 The 12 genes in the candidate interval on chromosome 4.
| 登录号 | 位置 | 功能注释 |
|---|---|---|
| Gene accession | Location/bp | Functional annotation |
| LOC_Os04g08290 | 4 427 683-4 424 830 | ZOS4-04-C2H2锌指蛋白 ZOS4-04-C2H2 zinc finger protein |
| LOC_Os04g08300 | 4 433 624-4 433 839 | 反转录转座子蛋白,Ty3-gypsy亚类 Retrotransposon protein, putative, Ty3-gypsy subclass |
| LOC_Os04g08310 | 4 436 856-4 441 657 | 表达蛋白 Expressed protein |
| LOC_Os04g08320 | 4 444 004-4 441 881 | 假定蛋白YIF1B YIF1B, putative |
| LOC_Os04g08330 | 4 446 960-4 446 649 | 表达蛋白 Expressed protein |
| LOC_Os04g08340 | 4 452 696-4 455 350 | OsSigP3-假定的Ⅰ型信号肽酶同系物;采用假定的Ser/Lys催化二元体 OsSigP3-putative Type I signal peptidase homologue, employs a putative Ser/Lys catalytic dyad |
| LOC_Os04g08350 | 4 461 845-4 457 458 | 半胱氨酸合酶,叶绿体/染色质前体 Cysteine synthase, chloroplast/chromoplast precursor |
| LOC_Os04g08360 | 4 463 046-4 463 552 | 表达蛋白 Expressed protein |
| LOC_Os04g08370 | 4 473 512-4 470 642 | 富含亮氨酸的重复家族蛋白 Leucine rich repeat family protein |
| LOC_Os04g08390 | 4 490 101-4 484 997 | 富含亮氨酸的重复家族蛋白 Leucine rich repeat family protein |
| LOC_Os04g08400 | 4 497 789-4 503 741 | 反转录转座子蛋白 Retrotransposon protein |
| LOC_Os04g08410 | 4 513 883-4 508 804 | 含有ELM2结构域蛋白 ELM2 domain containing protein |
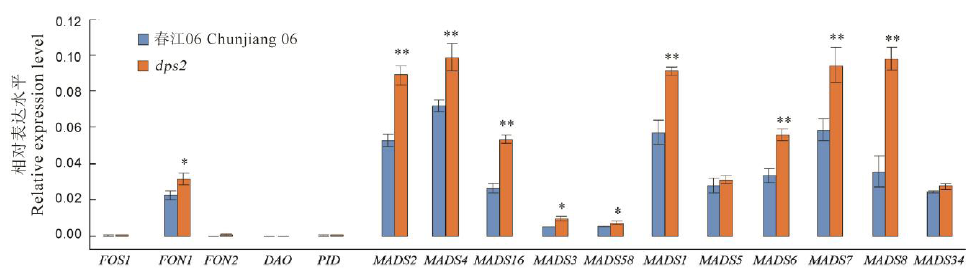
图6 野生型春江06与突变体dps2中水稻花器官数目决定和发育相关基因的表达误差线表示3次生物学重复试验的标准差。*和**分别表示野生型春江06和突变体dps2间差异达0.05和0.01显著水平(t检验)。
Fig. 6. Expression profile of the genes involved in the number and development of flower organs of dps2 mutant and wild type Chunjiang 06. Mean±SD(n=3). * and ** indicate the difference between the wild type Chunjiang 06 and mutant dps2 is significant at 0.05 and 0.01 levels, respectively according to Student’s t test.
| [1] | Pelaz S, Ditta G S, Baumann E, Wisman E, Yanofsky M F.B and C floral organ identity functions require SEPALLATA MADS-box genes[J]. Nature, 2000, 405(6783): 200-203. |
| [2] | Coen E S, Meyerowitz E M.The war of the whorls genetic interactions controlling flower development[J]. Nature, 1991, 353(6339): 31-37. |
| [3] | Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky M F.The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity[J]. Current Biology, 2004, 14(21): 1935-1940. |
| [4] | Causier B, Schwarz-Sommer Z, Davies B.Floral organ identity: 20 years of ABCs[J]. Seminars in Cell and Developmental Biology, 2010, 21(1): 73-79. |
| [5] | Kalika P, Usha V.Double-stranded RNA interference of a rice PI/GLO paralog, OsMADS2, uncovers its second-whorl-specific function in floral organ patterning[J]. Genetics,2003, 165(4): 2301-2305. |
| [6] | Shri R Y, Kalika P, Usha V.Divergent regulatoryOsMADS2 functions control size, shape and differentiation of the highly derived rice floret second-whorl organ. Genetics, 2007, 176(1): 283. |
| [7] | Shan G Y, Shinnosuke O, Mayumi K, Hitoshi Y.Unequal genetic redundancy of rice PISTILLATA orthologs, OsMADS2 and OsMADS4, in lodicule and stamen development[J]. Plant & Cell Physiology, 2008, 49(5): 853. |
| [8] | Nagasawa M, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y.SUPERWOMAN1 and DROOPING LEAF genes control floral organ identify in rice[J]. Development, 2003, (130): 708-718 |
| [9] | Yun D P, Liang W Q, Dreni L D, Yin C S, Zhou Z G, Kater M M, Zhang D B.OsMADS16 genetically interacts with OsMADS3and OsMADS58 in specifying floral patterning in rice[J]. Molecular Plant, 2013, 6(3): 743-756. |
| [10] | Hu Y, Liang W Q, Yin C S, Yang X L, Ping B Z, Li A X, Jia R, Chen M J, Luo Z J, Cai Q, Zhao X X, Zhang D B, Yuan Z.Interactions of OsMADS1 with floral homeotic genes in rice flower development[J]. Molecular Plant, 2015, 8(9): 1366-1384. |
| [11] | Hu L F, Liang W Q, Yin C S, Cui X, Zong J, Wang X, Hu J P, Zhang D B.Rice MADS3 regulates ROS homeostasis during late anther development[J]. Plant Cell, 2011, 23(2): 515-533. |
| [12] | Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano H Y.The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa[J]. Plant Cell, 2004, 16(2): 500-509. |
| [13] | Lopez-Dee ZP, Wittich P, Enrico P M, Rigola D, Del B I, Gorla M S, Kater M M, Colombo L.OsMADS13, a novel rice MADS-box gene expressed during ovule development[J]. Developmental Genetics, 2015, 25(3): 237-244. |
| [14] | Dreni L, Jacchia S, Fornara F, Fornari M, Ouwerkerk P B, An G, Colombo L, Kater M M.The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice[J]. The Plant Journal, 2007, 52(4): 690-699. |
| [15] | Prasad K, Sriram P, Kumar C S, Kushalappa K, Vijayraghavan U.Ectopic expression of rice OsMADS1 reveals a role in specifying the lemma and palea, grass floral organs analogous to sepals[J]. Development Genes & Evolution, 2001, 211(6): 281-290. |
| [16] | Li H F, Liang W Q, Rui D J, Yin C S, Zong J, Kong H Z, Zhang D B.The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice[J]. Cell Research, 2010, 20(3): 299. |
| [17] | Cui R F, Han J K, Zhao S Z, Su K M, Feng W, Du X Q, Xu Q J, Kang C, Theissen G, Zheng M.Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa)[J]. Plant Journal, 2010, 61(5): 767-781. |
| [18] | Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano H Y.The gene FLORAL ORGAN NUMBER1 regulates floral meristern size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1[J]. Development, 2004, 131(22): 5649-5657. |
| [19] | Suzaki T, Toriba T, Fujimoto M, Tsutsumi N, Kitano H, Hirano H Y.Conservation and diversification of meristem maintenance mechanism in Oryza sativa: Function of the FLORAL ORGAN NUMBER2 gene[J]. Plant & Cell Physiology, 2006, 47(12): 1591-1602. |
| [20] | Li J, Zhang W L, Xia Z H, Jiang G H, Qian Q, Li A L, Cheng Z K, Zhu L H, Long M, Zhai W X.A paracentric inversion suppresses genetic recombination at the FON3 locus with breakpoints corresponding to sequence gaps on rice chromosome 11L[J]. Molecular Genetics & Genomics ,2007, 277(3): 263-272. |
| [21] | 张向前, 邹金松, 朱海涛, 李晓燕, 曾瑞珍. 水稻早熟多子房突变体fon5的遗传分析和基因定位[J]. 遗传, 2008, 30(10): 1349-1355. |
| Zhang X Q, Zou J S, Zhu H T, Li X Y, Zeng R Z.Genetic analysis and gene mapping of an early flowering and multi-ovary mutant in rice (Oryza sativa L.)[J]. Hereditas, 2008, 30(10): 1349-1355. | |
| [22] | 赵福永,王洁雅, 黄显波, 邓则勤, 林成豹, 严寒, 田志宏. 水稻花器官数目突变体fon6的研究初报[J]. 杂交水稻, 2011, 26(2): 52-57. |
| Zhao F Y, Wang J Y, Huang X B, Deng Z Q, Lin C B, Han Y, Tian Z H.A preliminary study on the floral organ number mutant fon6 in rice[J]. Hybrid Rice, 2011, 26(2): 52-57. | |
| [23] | Thomson B, Zheng B, Wellmer F.Floral organogenesis: When knowing your ABCs is not enough[J]. Plant Physiol, 2017, 173(1): 56-64. |
| [24] | Livak K J, Schmittgen T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408. |
| [25] | Zhang D B, Wilson Z A.Stamen specification and anther development in rice[J]. Chinese Science Bulletin, 2009, 54(14): 2342-2353. |
| [26] | Xing Y Z, Zhang Q F.Genetic and molecular bases of rice yield[J]. Annual Review of Plant Biology, 2010, 61(1): 421-442. |
| [27] | Ikeda K, Sunohara H, Nagato Y.Development course of inflorescence and spikelet in rice[J]. Breeding Science, 2004, 54: 147-156. |
| [28] | 吴华茂. 水稻穗粒数相关QTL分析及水稻花器官发育突变体dps1的鉴定[D]. 中国科学院大学,2018. |
| Wu H M.Studies on rice QTL of grain number per panicle and mutant dps1 defective in floral organ development[D]. University of Chinese Academy of Science, 2018. | |
| [29] | Yamaguchi T, Lee D Y, Miyao A, Hirochika H, An G, Hirano H Y.Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa[J]. Plant Cell, 2006, 18(1): 15-28. |
| [30] | Moreau F, Thévenon E, Blanvillain R, Lopezvidriero I, Francozorrilla J M, Dumas R, Parcy F, Morel P, Trehin C, Carles C C.The Myb-domain protein ULTRAPETALA1 INTERACTING FACTOR 1 controls floral meristem activities in Arabidopsis[J]. Development, 2016, 143(7): 1108-1119. |
| [31] | Prunet N, Yang W B, Das P, Meyerowitz E M, Jack T P.SUPERMAN prevents class B gene expression and promotes stem cell termination in the fourth whorl of Arabidopsis thaliana flowers[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(27): 7166. |
| [32] | Sharma N, Xin R J, Kim D H, Sung S, Lange T, Huq E.NO FLOWERING IN SHORT DAY (NFL) is a bHLH transcription factor that promotes flowering specifically under short-day conditions in Arabidopsis[J]. Development, 2016, 143(4): 682. |
| [33] | Hugouvieux V, Silva C S, Jourdain A, Stigliani A, Charras Q, Conn V, Conn S J, Carles C C, Parcy F, Zubieta C.Tetramerization of MADS family transcription factors SEPALLATA3 and AGAMOUS is required for floral meristem determinacy in Arabidopsis[J]. Nucleic Acids Research, 2018. |
| [34] | Sommer R J, Retzlaff M, Goerlich K, Sander K, Tautz D.Evolutionary conservation pattern of zinc-finger domains of Drosophila segmentation genes[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(22): 10782-10786. |
| [35] | Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M.The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers[J]. Febs Letters, 2002, 514(2): 351-354. |
| [36] | Sakai H, Medrano LJ, Meyerowitz E M.Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries[J]. Nature, 1995, 378(6553): 199-203. |
| [37] | Gaiser J C, Robinson-Beers K, Gasser C S.The Arabidopsis SUPERMAN gene mediates asymmetric growth of the outer integument of ovules[J]. Plant Cell, 1995, 7(3): 333-345. |
| [38] | Nibau C, Stilio V S D, Wu H M, Cheung A Y. Arabidopsis and Tobacco SUPERMAN regulate hormone signalling and mediate cell proliferation and differentiation[J]. Journal of Experimental Botany, 2011, 62(3): 949-961. |
| [39] | Uemura A, Yamaguchi N, Xu Y, Wee W Y, Ichihashi Y, Suzuki T, Shibata A, Shirasu K, Ito T.Regulation of floral meristem activity through the interaction of AGAMOUS, SUPERMAN, and CLAVATA3 in Arabidopsis[J]. Plant Reproduction, 2017, 31(1): 89-105. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||