
中国水稻科学 ›› 2018, Vol. 32 ›› Issue (6): 519-528.DOI: 10.16819/j.1001-7216.2018.8023
• • 下一篇
吕凤1, 唐倩莹1, 王启明1, 郑海1, 尤世民1, 柏文婷1, 肖晏嘉1, 赵志刚1,*( ), 万建民1,2
), 万建民1,2
收稿日期:2018-03-09
修回日期:2018-04-03
出版日期:2018-11-27
发布日期:2018-11-10
通讯作者:
赵志刚
基金资助:
Feng LÜ1, Qianying TANG1, Qiming WANG1, Hai ZHENG1, Shimin YOU1, Wenting BAI1, Yanjia XIAO1, Zhigang ZHAO1,*( ), Jianmin WAN1,2
), Jianmin WAN1,2
Received:2018-03-09
Revised:2018-04-03
Online:2018-11-27
Published:2018-11-10
Contact:
Zhigang ZHAO
摘要:
目的 对水稻胚囊突变体的表型观察、遗传定位和基因克隆,将为研究植物生殖发育奠定理论基础。方法 从粳稻品种宁粳4号突变体库中筛选出一个雌性败育突变体female abortion(fa),对野生型和突变体不同发育时期的胚囊进行细胞学观察并统计胚囊类型。以突变体杂合型为母本,N22为父本构建定位群体,对该表型进行遗传分析,采用图位克隆方法精确定位目的基因。结果 表型分析显示,突变体雌蕊在外观上没有表现出差异,但成熟时植株完全不育。与野生型相比, 该突变体胚囊发育异常,不能形成七细胞八核胚囊结构,而且形成多种类型的异常胚囊。遗传分析表明,该突变性状受一对隐性核基因控制。我们将该基因初定位在第1染色体的分子标记L1和L3之间,通过扩大定位群体,最终将基因定位在分子标记L10和L11之间,物理距离为117 kb。测序分析发现该区间内LOC_Os01g68870外显子上有一个单碱基替换,由胸腺嘧啶(T)替换成胞嘧啶(C),氨基酸由亮氨酸变为脯氨酸,从而导致该表型的出现。qRT-PCR结果显示,相对于OsMADS13、OsAPC6、OsTDL1A基因在突变体fa胚囊中表达量而言,OsDEES1基因在突变体fa胚囊中表达量变化最为显著,FA可能是OsDEES1的上游基因。亚细胞定位结果显示,该蛋白定位于质膜。结论 FA是多孢囊基因MULTIPLE SPOROCYTE 1(MSP1)的新等位基因。本研究进一步明确了该基因对于水稻胚囊发育的重要性,可为探明它所在的调控网络体系提供新的线索。
中图分类号:
吕凤, 唐倩莹, 王启明, 郑海, 尤世民, 柏文婷, 肖晏嘉, 赵志刚, 万建民. 水稻雌性败育基因FA的图位克隆[J]. 中国水稻科学, 2018, 32(6): 519-528.
Feng LÜ, Qianying TANG, Qiming WANG, Hai ZHENG, Shimin YOU, Wenting BAI, Yanjia XIAO, Zhigang ZHAO, Jianmin WAN. Map-based Cloning of Female Abortion (FA) Gene in Rice[J]. Chinese Journal OF Rice Science, 2018, 32(6): 519-528.
| 正向引物 Forward sequence(5'-3') | 反向引物序列 Reverse sequence(5'-3') | 实验目的 Purpose |
|---|---|---|
| ACGCTGAAAAGCAAGGATGT | TTCTAGCCCTCCTCTTTGACA | 初定位 Preliminary mapping |
| AATTCGGTCACTCGCTGTCACG | CTGCGGACGAAATTGCTTAGCC | 初定位 Preliminary mapping |
| GGGATTATTTGAAATCTTTGC | ATATAGCATTGCCAGTTTGC | 初定位 Preliminary mapping |
| GTCGACGGCTTCCTCAAGATTGG | TGAGACCTCTGTGAAGGCACTCG | 初定位 Preliminary mapping |
| CGAGTTCGTCCAAGTGAGC | CATCCACCATTCCACCAATC | 精细定位Fine maping |
| GAAAGGTGATGGGAGAGCAG | TGACACCCTCTCTCCACCTC | 精细定位Fine maping |
| AAGCCAGCAATGTTATGAGC | CATTACCAGCAGCGGAGTA | 精细定位Fine maping |
| CATGGGCCAGAATTAAGAGG | CATCCACTTTCCTCTCCTGC | 精细定位Fine maping |
| GCATGCTACCACCTTGACTGC | GTGAGTAGCGAGACCGAGAGTGC | 精细定位Fine maping |
| AGCTGTGCGAGGAATCCAAACG | CTCCGATCCCAGCAGCTACTCC | 精细定位Fine maping |
| GTTATATGGCTCTGGCACGC | TTGTTTAGGACACCGCTTGC | 精细定位Fine maping |
| TGTTGTGCAAGTGATTGGCA | CAAGACACATCGTACCGCTG | 精细定位Fine maping |
| TTGCTCCTCCCTACAACAGT | AGCTATGGCAAGAAGAGGTGA | 精细定位Fine maping |
| TGGTTACCTGCCTTGATCGA | CTTTCTGCTCCGTTTGTTGC | 精细定位Fine maping |
| TGTGGCTGAGAAACCGAGCA | CGACTTCATGTCCCGGTGGA | qRT-PCR |
| ACGACGACTGCCTCCTCAAC | GAGGCGTAGCAAATGGTGAG | qRT-PCR |
| TGCCAGGAATAAGTCTGCTG | TAATACTTCGGCAAGCAACG | qRT-PCR |
| CCCGGTAAGTGAGGGTACAA | TGATCCAAAACCACTCCAGA | qRT-PCR |
表1 本研究定位和定量PCR所用引物序列
Table 1 Primer for gene mapping and qRT-PCR.
| 正向引物 Forward sequence(5'-3') | 反向引物序列 Reverse sequence(5'-3') | 实验目的 Purpose |
|---|---|---|
| ACGCTGAAAAGCAAGGATGT | TTCTAGCCCTCCTCTTTGACA | 初定位 Preliminary mapping |
| AATTCGGTCACTCGCTGTCACG | CTGCGGACGAAATTGCTTAGCC | 初定位 Preliminary mapping |
| GGGATTATTTGAAATCTTTGC | ATATAGCATTGCCAGTTTGC | 初定位 Preliminary mapping |
| GTCGACGGCTTCCTCAAGATTGG | TGAGACCTCTGTGAAGGCACTCG | 初定位 Preliminary mapping |
| CGAGTTCGTCCAAGTGAGC | CATCCACCATTCCACCAATC | 精细定位Fine maping |
| GAAAGGTGATGGGAGAGCAG | TGACACCCTCTCTCCACCTC | 精细定位Fine maping |
| AAGCCAGCAATGTTATGAGC | CATTACCAGCAGCGGAGTA | 精细定位Fine maping |
| CATGGGCCAGAATTAAGAGG | CATCCACTTTCCTCTCCTGC | 精细定位Fine maping |
| GCATGCTACCACCTTGACTGC | GTGAGTAGCGAGACCGAGAGTGC | 精细定位Fine maping |
| AGCTGTGCGAGGAATCCAAACG | CTCCGATCCCAGCAGCTACTCC | 精细定位Fine maping |
| GTTATATGGCTCTGGCACGC | TTGTTTAGGACACCGCTTGC | 精细定位Fine maping |
| TGTTGTGCAAGTGATTGGCA | CAAGACACATCGTACCGCTG | 精细定位Fine maping |
| TTGCTCCTCCCTACAACAGT | AGCTATGGCAAGAAGAGGTGA | 精细定位Fine maping |
| TGGTTACCTGCCTTGATCGA | CTTTCTGCTCCGTTTGTTGC | 精细定位Fine maping |
| TGTGGCTGAGAAACCGAGCA | CGACTTCATGTCCCGGTGGA | qRT-PCR |
| ACGACGACTGCCTCCTCAAC | GAGGCGTAGCAAATGGTGAG | qRT-PCR |
| TGCCAGGAATAAGTCTGCTG | TAATACTTCGGCAAGCAACG | qRT-PCR |
| CCCGGTAAGTGAGGGTACAA | TGATCCAAAACCACTCCAGA | qRT-PCR |
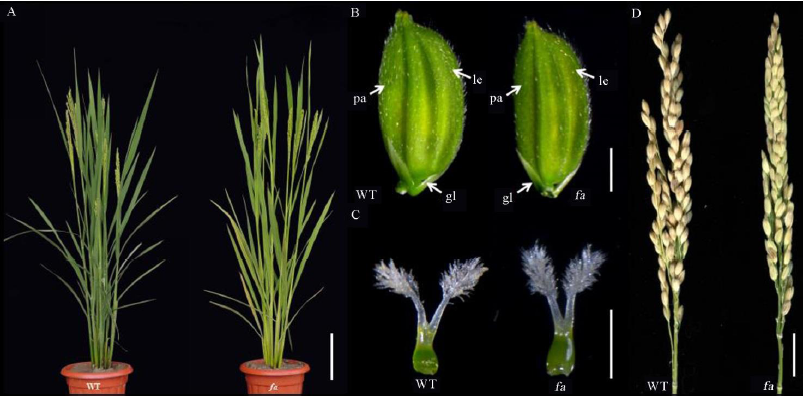
图1 野生型和fa 突变体表型^ A–野生型和突变体fa的株型和抽穗形态,标尺为20 cm。B–野生型和突变体fa颖花表型,标尺为2 mm。le–外稃;pa–内稃;gl–护颖。C–野生型和突变体fa的雌蕊表型,标尺为1 mm。D–野生型和突变体fa的成熟穗,标尺为2.5 cm。
Fig. 1. Phenotypes of the wild type and the fa mutant.^ A, Plant morphology of the wild type(WT) and the fa mutant during heading, bar=20 cm. B, Spikelet of WT and the fa mutant, bar=2 mm. le, Lemma; pa, Palea; gl, Glume. C, Pistil of WT and the fa mutant, bar=1 mm. D, Mature spikelet of WT and the fa mutant, bar=2.5 cm.
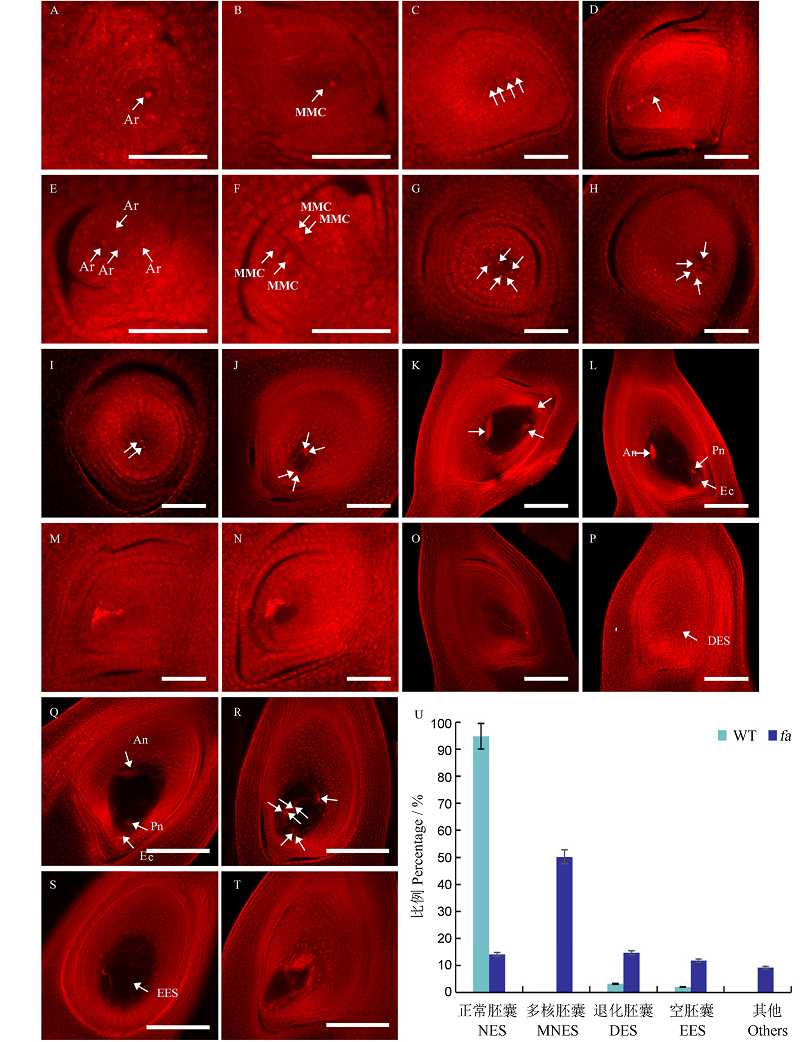
图2 野生型和fa 突变体的胚囊发育过程观察及其不同类型的胚囊统计^ A~D,I~L为野生型胚囊发育过程;E~H,M~T代表突变体fa胚囊发育过程。A和E–孢原细胞期;B和F–大孢子母细胞期;C和G–四分体期;D和H–功能大孢子期;I和M–二核胚囊期;J和N–四核胚囊期;K和O–八核胚囊期;L和P~T–成熟胚囊期。箭头指示核。U–统计野生型(n=156)和突变体fa(n=216)成熟胚囊中不同类型的胚囊。An–反足细胞;Ar–胞原细胞;Ec–卵细胞;MMC–大孢子母细胞;Pn–极核。A~P标尺为50 µm,Q~T标尺为100 µm。
Fig. 2. Embryo sac development of the wild type(WT) and fa mutant and statistics of different types of embryo sacs.^ A to D and I to L, Wild type; E to H and M to T, fa mutant. A and E, Archesporial cell stage; B and F, Megaspore mother cell stage; C and G, Tetrad stage; D and H, Functional megaspore stage; I and M, Bi-nuclear embryo sac; J and M, Tetra-nuclear embryo sac; K and O, Eight-nuclear embryo sac; L and P to T, Mature embryo sac. Arrows indicate nuclei. U, Statistics of different types of embryo sacs in mature embryo sac of the wild type (n=156) and the mutant (n=216). An, Antipode cell; Ar, Archesporial cell; Ec, Egg cell; MMC, Megaspores mother cell; Pn, Polar nuclei; NES, Normal embryo sac; MNES, Multi-nucleated embryo sac; DES, Degenerated embryo sac; EES, Empty sac with no embryo formation; Others, Other abnormal embryo sacs. Bar= 50 µm in A to P and 100 µm in Q to T.
| 杂交组合 Combination | 授粉的花数目 No. of flowers pollinated | 成熟种子数目 No. of mature seeds |
|---|---|---|
| fa×宁粳4 fa×Ningjing 4 fa×fa | 128 143 | 15 0 |
表2 突变体杂交组合授粉情况与成熟种子数目统计
Table 2 Combinations of the fa mutant and statistics of mature seeds.
| 杂交组合 Combination | 授粉的花数目 No. of flowers pollinated | 成熟种子数目 No. of mature seeds |
|---|---|---|
| fa×宁粳4 fa×Ningjing 4 fa×fa | 128 143 | 15 0 |
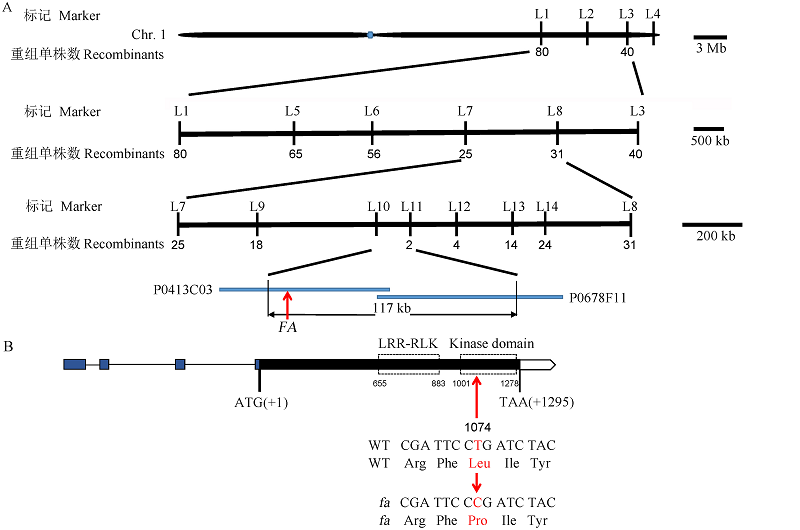
图3 FA基因在第1染色体上的分子定位及结构分析^ A–FA基因的精细定位,P0413C03和P0678F11是基因组BAC登录号,FA基因定位在两个分子标记L10和L11间的117 kb区间内;B–FA基因的结构,黑色框代表由起始密码子ATG(+1)开始到终止密码子TAA(+1295)结束的外显子,编码1294个氨基酸,第655个氨基酸到第883个氨基酸代表LRR-RLK (Leu-rich repeat receptor-like protein kinase)结构域,第1001到第1278个氨基酸代表激酶结构域。第1074个氨基酸由亮氨酸替换成脯氨酸。蓝色框代表5′非翻译区,白色框代表3′非翻译区。
Fig. 3. FA gene mapping on chromosome 1 and structural analysis.^ A, Fine mapping of the FA gene, P0413C03 and P0678F11 are genomic BAC accession numbers, and the FA gene is mapped to a 117-kb region between two molecular markers L10 and L11; B, Genomic structure of the FA gene, black box represent exon beginning with the start codon ATG (+1) and ending with the stop codon TAA (+1295), encoding 1294 amino acids, and amino acids 655 to 883 representing LRR-RLKs(Leu-rich repeat receptor-like protein kinase) domain, and the 1001th to 1278th amino acids represent a kinase domain. The 1074th amino acid leucine is replaced by proline. The blue box represents the 5'- untranslated region and the white box represents the 3'- untranslated region.
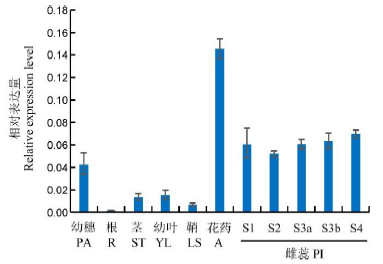
图4 FA基因在水稻各组织中的相对表达量^以OsUbiquitin作为对照,误差线表示n=3时的标准差。S1―孢原细胞形成期,S2―大孢子母细胞形成期,S3a―二分体时期,S3b-四分体时期,S4―功能大孢子形成期。
Fig. 4. Relative expression levels of FA in rice tissues. ^ PA, Panicle; R, Root; ST, Stem; YL, Young Leaf; LS, Leaf sheath; A, Anther; PI, Pistil. OsUbiquitin was used as an internal control, Error bars show SD (n=3). S1, Archesporial cell stage; S2, Megaspore mother cell stage; S3a, Dyad stage; S3b, Tetrad stage; S4, Functional megaspore stage.
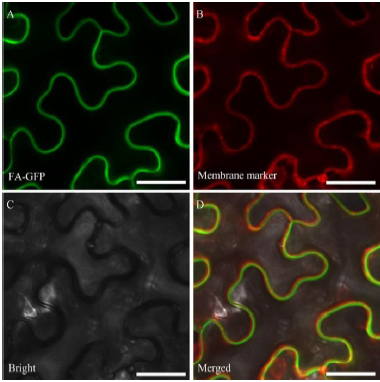
图5 FA蛋白在烟草中的亚细胞定位^ A–FA-GFP在烟草表皮细胞中的亚细胞定位;B–膜标签在烟草表细胞中的定位;C–明场视野下烟草表细胞状态;D–GFP、膜标签和明场的合并。GFP代表绿色荧光蛋白。标尺=100 µm。
Fig. 5. Subcellular localization of FA protein in tobacco. ^ A, Subcellular localization of FA-GFP in tobacco epidermal cells; B, Localization of membrane marker in tobacco epidermal cells; C, Bright field of tobacco epidermal cell status; D, The merging of GFP, membrane marker and bright field. GFP indicates the green fluorescence of proteins. Bar=100 µm.
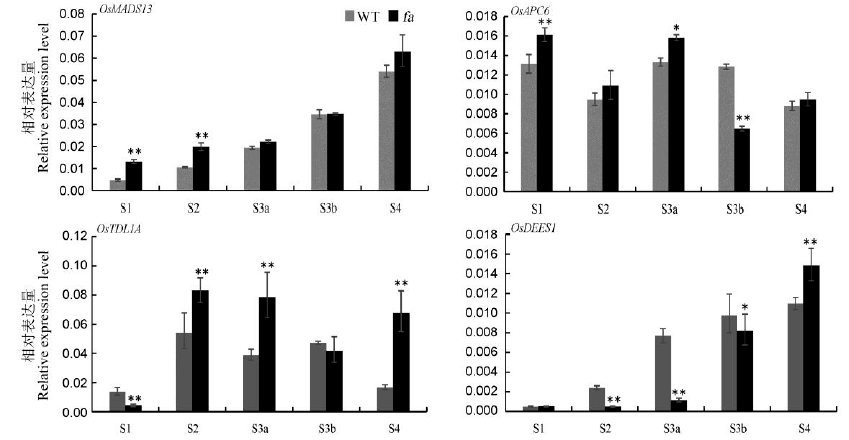
图6 野生型和fa突变体不同时期中胚囊相关基因的表达^野生型(WT)和突变体fa胚囊发育相关基因在不同时期的表达量。以OsUbiquitin作为对照,误差线表示n=3时的标准差。*和**分别代表野生型与突变体fa差异达0.05和0.01极显著水平(t测验)。S1―孢原细胞形成期,S2―大孢子母细胞形成期,S3a―二分体时期,S3b-四分体时期,S4―功能大孢子形成期。
Fig. 6. Expression of embryo sac related genes of the wild type and fa mutant at different stages.^ Expression levels of gene related to embryo sac development in the wild type (WT) and mutant fa at different stages. OsUbiquitin was used as an internal control; Error bars show SD (n=3). * and ** indicate significant difference between the wild type and fa mutant by t-test, respectively(P<0.05, P<0.01). S1, Archesporial cell stage; S2, Megaspore mother cell stage; S3a, Dyad stage; S3b, Tetrad stage; S4, Functional megaspore stage.
| [1] | Zhao L H, He J M, Lin H Y, Li Y Q, Liu R Y, Yang Z B, Qin Y.Comparative expression profiling reveals gene functions in female meiosis and gametophyte development in Arabidopsis. Plant Physiol, 2014, 80(4): 615-628. |
| [2] | Evans M M S. Theindeterminate gametophyte1 gene of maize encodes a LOB domain protein required for embryo sac and leaf development. Plant Cell, 2007, 19(1): 46-62. |
| [3] | Wang N, Huang H J, Ren S T, Li J J, Sun Y, Sun D Y, Zhang S Q.The rice wall-associated receptor-like kinase gene OsDEES1 plays a role in female gametophyte development. Plant Physiol, 2012, 160(2): 696-707. |
| [4] | 官文祥, 邓赟, 李小旭, 吴为人, 郑燕. 水稻雌性不育分子机理研究进展. 分子植物育种, 2017, 15(2): 672-684. |
| Guan W X, Deng Y, Li X X, Wu W R, Zheng Y.Advances in research on molecular mechanism of female sterility in rice (Oryza sativa L.). Mol Plant Breed, 2017, 15(2): 672-684. (in Chinese with English abstract) | |
| [5] | 凌定厚, 马镇荣, 陈梅芳, 陈琬瑛. 起源于体细胞培养的籼稻雌性不育突变. 遗传学报, 1991, 18(5): 446-451. |
| Ling D H, Ma Z H, Chen M F, Chen W Y.Female sterile mutant from somaclones in somatic cell culture of indica rice.Acta Genet Sin, 1991, 18(5): 446-451. (in Chinese with English abstract) | |
| [6] | Pelaz S, Ditta G S, Baumann E, Wisman E, Yanofsky M F.B and C floral organ identity function require SEPALLATA MADS-box genes.Nature, 2000, 405(6783): 200-203. |
| [7] | Dreni L, Jacchia S, Fornara F, Fornari M,Ouwerkerk P B F, An G, Colombo L,Kater M M. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J, 2007, 52(4): 690-699. |
| [8] | Li H F, Liang W Q, Yin C S, Zhu L, Zhang D B.Genetic interaction of OsMADS3, DROOPING LEAF, and OsMADS13 in specifying rice floral organ identities and meristem determinacy. Plant Physiol, 2011, 156(1): 263-274. |
| [9] | Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano H Y.The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell, 2004, 16(2): 500-509. |
| [10] | Nonomura K I, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N.The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell, 2003, 15(8): 1728-1739. |
| [11] | Zhao X A, Palma J D, Oane R, Gamuyao R, Luo M, Chaudhury A, Herve P, Xue Q Z, Bennett J.OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. Plant J, 2008, 54(3): 375-387. |
| [12] | Hong L L, Tang D, Shen Y, Hu Q, Wang K J, Li M, Lu T G, Cheng Z K.MIL2 (MICROSPORELESS2) regulates early cell differentiation in the rice anther.New Phytol, 2012, 196(2): 402-413. |
| [13] | Li Y, Qian X L, Chen M J, Fei Q L, Meyers B C, Liang W Q, Zhang D B.Regulatory role of a receptor-like kinase in specifying anther cell identity.Plant Physiol, 2016, 171(3): 2085-2100. |
| [14] | Kumar M, Basha P O, Puri A, Rajpurohit D, Randhawa G S, Sharma T R, Dhaliwal H S.A candidate gene OsAPC6 of anaphase-promoting complex of rice identified through T-DNA insertion. Funct Integr Genom, 2010, 10(3): 349-358. |
| [15] | Awasthi A, Paul P, Kumar S, Verma S K, Prasad R, Dhaliwal H S.Abnormal endosperm development causes female sterility in rice insertional mutantOsAPC6. Plant Sci, 2012, 183: 167-174. |
| [16] | Rogers S O, Bendich A J.Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues.Plant Mol Biol, 1985, 5(2): 69-76. |
| [17] | 刘向东, 徐雪宾, 卢永根, 徐是雄. 水稻胚囊形成过程与分期. 中国水稻科学, 1997, 11(3): 141-150. |
| Liu X D, Xu X B, Lu Y G, Xu S X.The proeess of embryo sac formation and its stages dividing in rice.Chin J Rice Sci, 1997, 11(3): 141-150. (in Chinese with English abstract) | |
| [18] | Shiu S H, Bleecker A B.Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA, 2001, 98(19): 10 763-10 768. |
| [19] | Torii K U, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier R F, Komeda Y.The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell, 1996, 8(6): 735-746. |
| [20] | Kim C H, Jeong D H, An G.Molecular cloning and characterization of OsLRK1 encoding a putative receptor-like protein kinase from Oryza sativa. Plant Sci, 2000, 152(1): 17-26. |
| [21] | Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano H Y, The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development, 2004, 131(22): 5649-5657. |
| [22] | Pu C X, Han Y F, Zhu S, Song F Y, Zhao Y, Wang C Y, Zhang Y C, Yang Q, Wang J, Bu S L, Sun L J, Zhang S W, Zhang S Q, Sun D Y, Sun Y.The rice receptor-like kinases DWARF AND RUNTISH SPIKELET1 and 2 repress cell death and affect sugar utilization during reproductive development.Plant Cell, 2017, 29(1): 70-89. |
| [23] | Yu J P, Han J J, Kim Y J, Song M, Yang Z, He Y, Fu R F, Luo Z J, Hu J P, Liang W Q, Zhang D B.Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc Natl Acad Sci USA, 2017, 114(46): 12327-12332. |
| [24] | Canales C, Bhatt A M, Scott R, Dickinson H.EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis.Curr Biol, 2002, 12(20): 1718-1727. |
| [25] | Zhao D Z, Wang G F, Speal B, Ma H.The EXCESS MICROSPOROCYTES1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev, 2002, 16(15): 2021-2031. |
| [26] | Shin H S, Bleecker A B.Plant receptor-like kinase gene family: Diversity, function, and signaling.Sci STKE, 2001, 113: 22. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||