
中国水稻科学 ›› 2016, Vol. 30 ›› Issue (5): 447-457.DOI: 10.16819/j.1001-7216.2016.6048
• • 下一篇
潘鹏屹, 朱建平, 王云龙, 郝媛媛, 蔡跃, 张文伟, 江玲, 王益华, 万建民*( )
)
收稿日期:2016-03-19
修回日期:2016-04-28
出版日期:2016-09-10
发布日期:2016-09-10
通讯作者:
万建民
基金资助:
Peng-yi PAN, Jian-ping ZHU, Yun-long WANG, Yuan-yuan HAO, Yue CAI, Wen-wei ZHANG, Ling JIANG, Yi-hua WANG, Jian-min WAN*( )
)
Received:2016-03-19
Revised:2016-04-28
Online:2016-09-10
Published:2016-09-10
Contact:
Jian-min WAN
摘要:
从甲基亚硝基脲(1-Methyl-1-Nitrosourea, MNU)处理的粳稻品种滇粳优1号突变体库中,筛选到一个稳定遗传的胚乳粉质突变体ws,其籽粒的千粒重、籽粒大小、总淀粉含量、直链淀粉含量等指标均降低,淀粉在尿素溶液中的膨胀能力减弱。对成熟及发育中的胚乳淀粉结构进行观察,发现ws突变体的胚乳中产生大量小而不规则排布的单淀粉颗粒。利用F2群体中分离出的92个隐性极端个体将突变基因连锁在第8染色体近着丝粒位置,随后共用2025个极端个体将目标基因定位于95 kb的区间。测序发现ws突变体中编码腺苷二磷酸葡萄糖焦磷酸化酶(Adenosine diphosphate glucose pyrophosphorylase, AGPase)小亚基S2的基因发生点突变,导致编码氨基酸的替换。基因表达分析发现,突变体胚乳中编码AGPase各亚基的相关基因表达量没有发生显著改变,而Western杂交分析显示突变体中AGPS2b的蛋白含量下降。同时,ws突变体的胚乳中AGPase活性下降为野生型的一半。研究结果表明,OsAGPS2的突变导致水稻胚乳中AGPase活性降低,从而影响了淀粉合成。
中图分类号:
潘鹏屹, 朱建平, 王云龙, 郝媛媛, 蔡跃, 张文伟, 江玲, 王益华, 万建民. 水稻粉质胚乳突变体ws的表型分析及基因克隆[J]. 中国水稻科学, 2016, 30(5): 447-457.
Peng-yi PAN, Jian-ping ZHU, Yun-long WANG, Yuan-yuan HAO, Yue CAI, Wen-wei ZHANG, Ling JIANG, Yi-hua WANG, Jian-min WAN. Phenotyping and Gene Cloning of a Floury Endosperm Mutant ws in Rice[J]. Chinese Journal OF Rice Science, 2016, 30(5): 447-457.
| 标记 Marker | 正向引物Forward (5'→3') | 反向引物Reverse (5'→3') |
|---|---|---|
| HY8-19 | TTTGTTGCTTTTCTGATTC | ATGATAAAGCGATAAACCA |
| WQ8-28 | GAGACGGACGGGTGTTGA | CAATGACATCCCAGCGTA |
| WZ8-17 | TAAATCATGGTGGTGGGC | ACCGTCGTCTAGCAAGGAG |
| WZ8-3 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| HY8-24 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
表1 WS基因定位引物的序列
Table 1 Markers for fine mapping of WS.
| 标记 Marker | 正向引物Forward (5'→3') | 反向引物Reverse (5'→3') |
|---|---|---|
| HY8-19 | TTTGTTGCTTTTCTGATTC | ATGATAAAGCGATAAACCA |
| WQ8-28 | GAGACGGACGGGTGTTGA | CAATGACATCCCAGCGTA |
| WZ8-17 | TAAATCATGGTGGTGGGC | ACCGTCGTCTAGCAAGGAG |
| WZ8-3 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| HY8-24 | ATTAAGATGATATGGGAAGT | ACATTGACCTGGTAGAAAC |
| 基因 Gene | 正向引物 Forward (5'→3') | 反向引物 Reverse (5'→3') |
|---|---|---|
| AGPL1 | CATCAAGGACGGGAAGGTCA | ACTTCACTCGGGGCAGCTTA |
| AGPL2 | CTGAGGAAGAGGTGCTTTGG | TCTTTCGGGAGGATTGTGTC |
| AGPS1 | AGAATGCTCGTATTGGAGAAAATG | GGCAGCATGGAATAAACCAC |
| AGPS2a | ACTCCAAGAGCTCGCAGACC | GCCTGTAGTTGGCACCCAGA |
| AGPS2b | AACAATCGAAGCGCGAGAAA | GCCTGTAGTTGGCACCCAGA |
| UGPase1 | CCATCACCGCCAAGTCA | GACCGTTGATGTCCTTGTTCT |
| Actin | CCCTCCTGAAAGGAAGTACAGTGT | GTCCGAAGAATTAGAAGCATTTCC |
表2 实时RT-PCR引物序列
Table 2 Primers used in real-time RT-PCR.
| 基因 Gene | 正向引物 Forward (5'→3') | 反向引物 Reverse (5'→3') |
|---|---|---|
| AGPL1 | CATCAAGGACGGGAAGGTCA | ACTTCACTCGGGGCAGCTTA |
| AGPL2 | CTGAGGAAGAGGTGCTTTGG | TCTTTCGGGAGGATTGTGTC |
| AGPS1 | AGAATGCTCGTATTGGAGAAAATG | GGCAGCATGGAATAAACCAC |
| AGPS2a | ACTCCAAGAGCTCGCAGACC | GCCTGTAGTTGGCACCCAGA |
| AGPS2b | AACAATCGAAGCGCGAGAAA | GCCTGTAGTTGGCACCCAGA |
| UGPase1 | CCATCACCGCCAAGTCA | GACCGTTGATGTCCTTGTTCT |
| Actin | CCCTCCTGAAAGGAAGTACAGTGT | GTCCGAAGAATTAGAAGCATTTCC |

图1 野生型与ws突变体的表型比较 A-野生型与ws突变体种子表型比较,标尺为3 mm; B-野生型与ws突变体种子横切面,标尺为1 mm; C-野生型与ws突变体植株,标尺为20 cm。DJY-滇粳优1号(野生型)。
Fig. 1. Phenotype comparison of the wild-type and ws mutant. A, Comparison of wild-type and ws mutant seeds, bar = 3 mm; B, Seed cross-sections of wild-type and ws mutant, bar = 1 mm; C, Comparison of wild-type and ws mutant plants, bar = 20 cm. DJY, Dianjingyou 1(wild type).
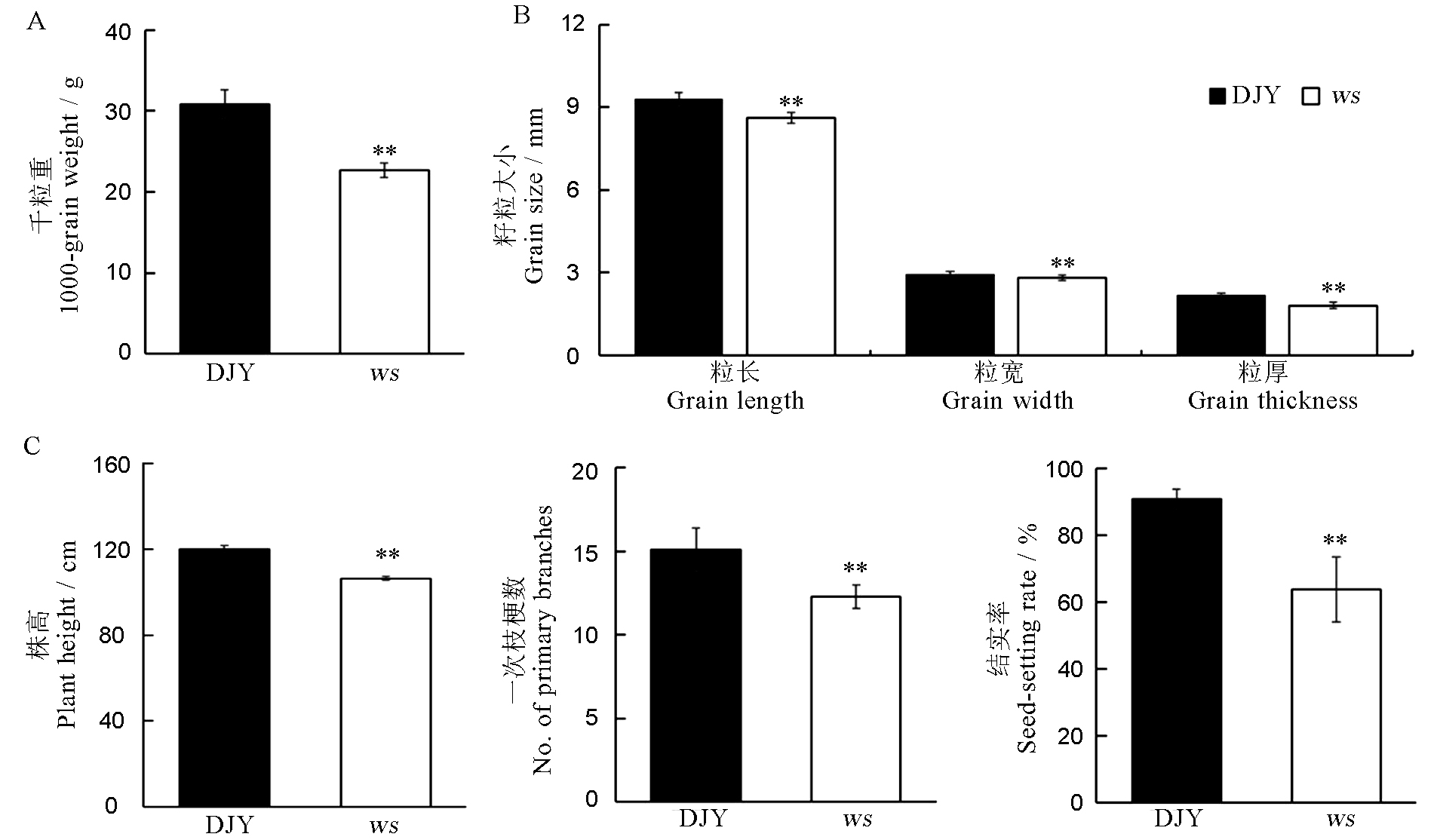
图2 野生型与ws突变体的千粒重、籽粒及主要农艺性状比较 千粒重n = 3,其余n = 10,取平均值± SD; 采用t测验,**表示P < 0.01。DJY-滇粳优1号(野生型)。
Fig. 2. Comparison of 1000-grain weight, seed and major agronomic traits of wild-type and ws mutant. Values are mean ± SD (n = 10, except for 1000-grain weight, n = 3); t-test, ** P < 0.01. DJY, Dianjingyou 1(wild type).
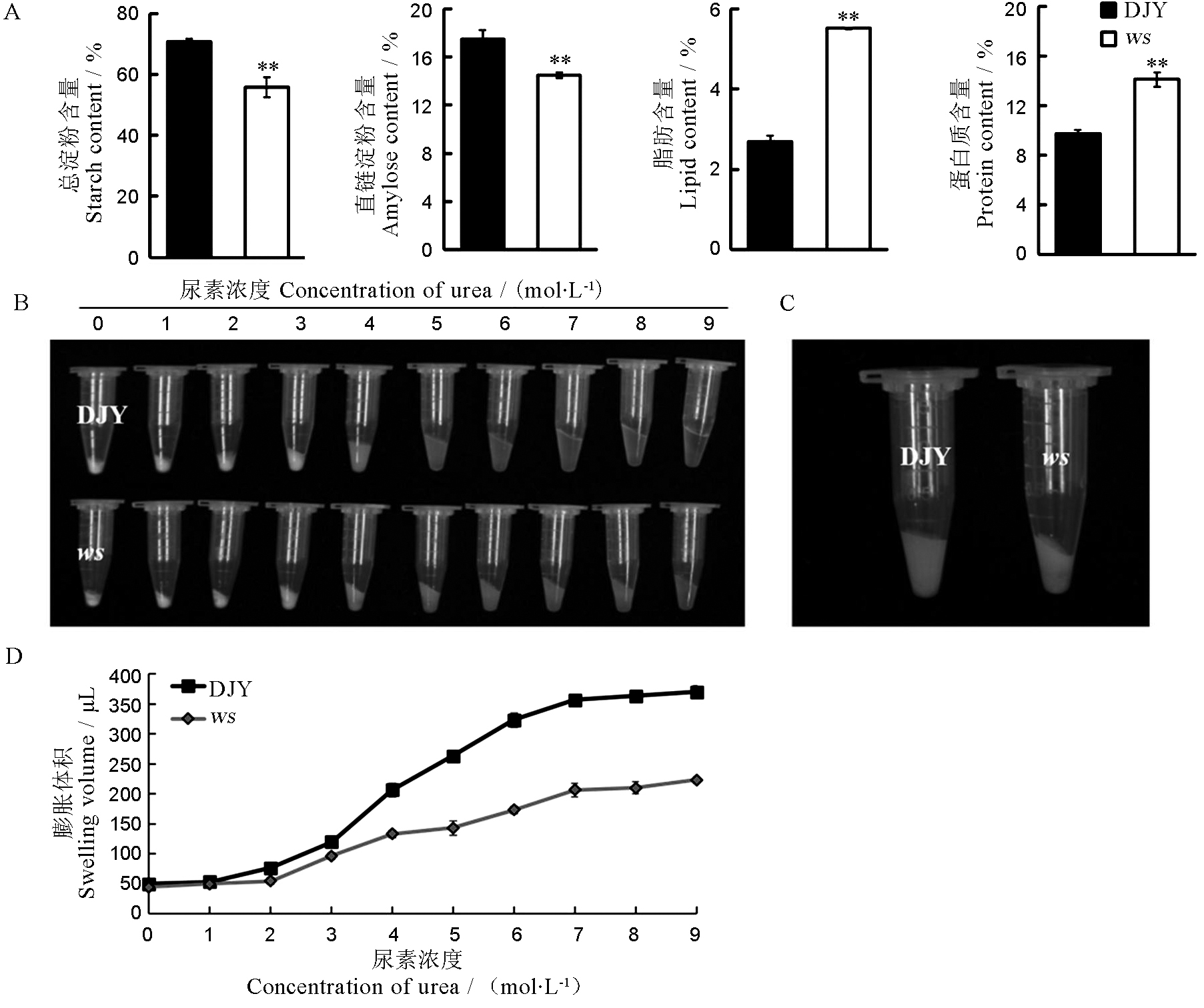
图3 野生型与ws突变体成熟籽粒的理化性质分析 A-野生型与ws突变体籽粒的化学成分分析(Mean± SD),n = 3,**,P < 0.01(t测验); B-野生型与ws突变体的尿素膨胀; C-4 mol/L的尿素浓度下出现显著差异; D-不同尿素浓度下野生型和突变体的米粉的膨胀体积比较(n = 3)。DJY-滇粳优1号(野生型)。
Fig. 3. Physicochemical characteristics of wild-type and ws mature seeds. A, Comparison of chemical composition of wild-type and ws seeds, values are mean ± SD, n = 3; t-test, ** P < 0.01; B, Gelatinization properties of wild type and ws mutant seeds; C, Significant difference was observed at the urea concentration of 4 mol/L urea; D, The swollen volume of wild-type and ws starch in urea solutions of various concentrations (n = 3). DJY, Dianjingyou 1(wild type).
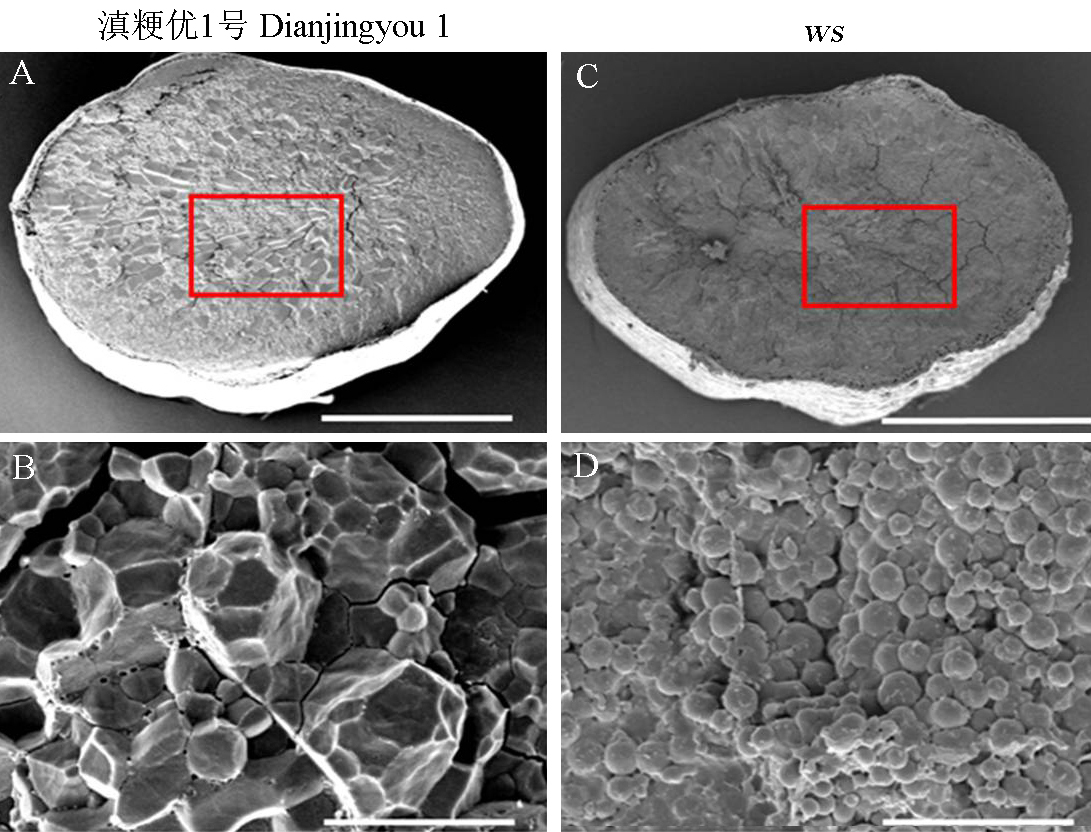
图4 野生型与ws突变体成熟籽粒的扫描电镜观察 A,B-野生型胚乳; C,D-ws突变体胚乳。A,C为籽粒横切面,标尺为1 mm; B,D是局部放大后的胚乳淀粉结构(红色方框内),标尺为10 μm。
Fig. 4. Scanning electron microscopy observation of mature seeds of wild type and ws mutant. A, B, Endosperm of wild-type; C, D, Endosperm of ws mutant; A, C, Endosperm cross-section, Bars = 1 mm; B, D, Partial enlarged drawing picture, Bars = 10 μm.

图5 野生型与ws突变体胚乳的半薄切片观察 A,B-开花后9 d野生型胚乳细胞外围、里层; C-开花后12 d野生型胚乳细胞里层; D,E-开花后9 d的ws突变体胚乳细胞外围、里层; F-开花后12 d的ws突变体胚乳细胞里层。A,D标尺是100 μm,B,C,E,F标尺是50 μm。
Fig. 5. Semi-thin sections of wild type and ws mutant seeds. A, B, Peripheral part (A) and central part (B) of wild-type endosperm cells at 9 DAF (days after flowering); C, Central part of wild-type endosperm cells at 12 DAF; D, E, Peripheral part (D) and central part (E) of ws endosperm cells at 9 DAF; F, Central part of ws endosperm cells at 12 DAF. Bars = 100 μm in A and D, 50 μm in B, C, E, F.
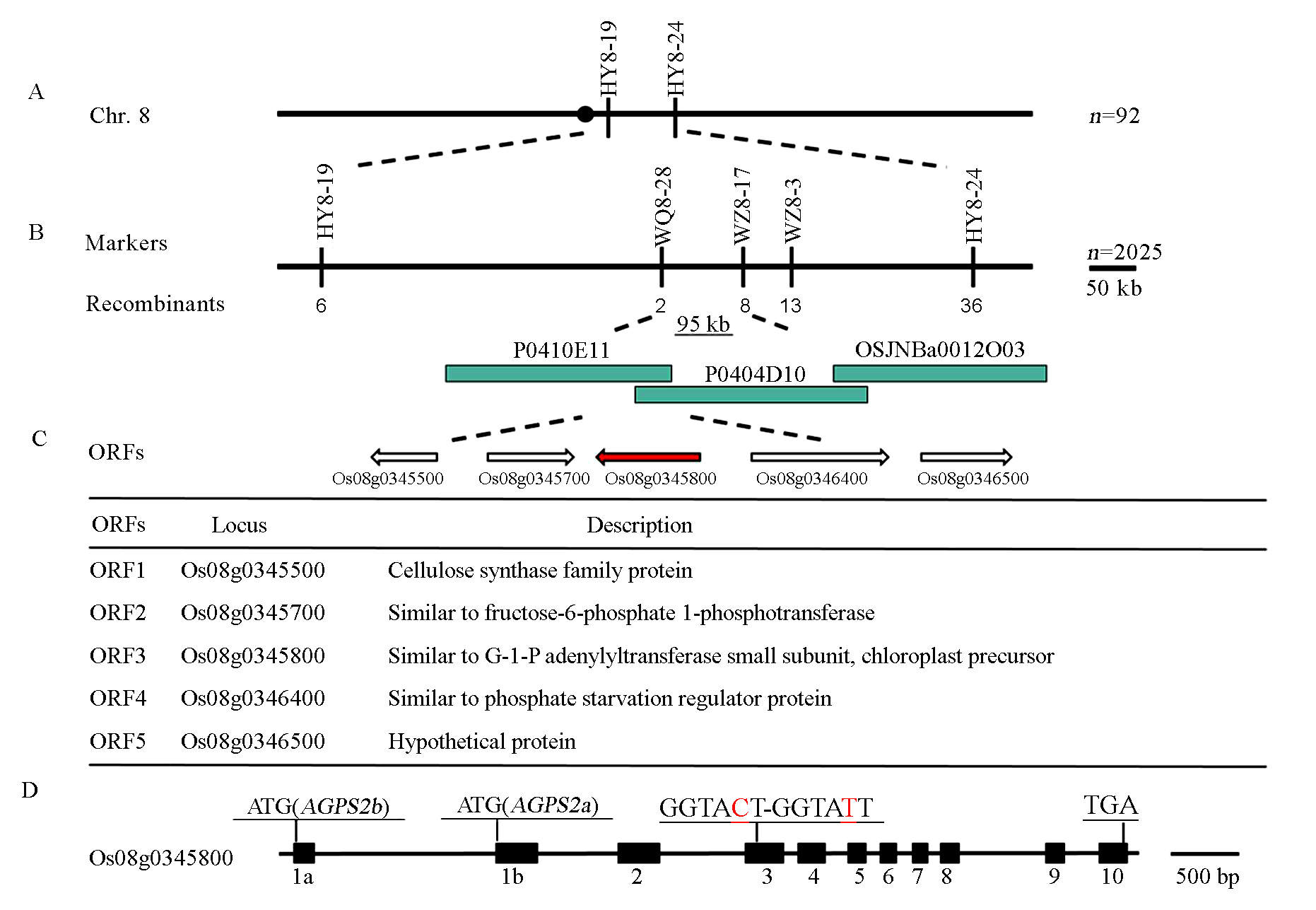
图6 WS基因的精细定位 A-WS基因与标记HY8-19和HY8-24连锁; B-利用2025个极端个体将WS基因定位在95 kb区间内; C-WS候选基因分析; D-突变体AGPS2中发生单碱基替换(红色标出),AGPS2由10个外显子(黑色方框表示)和9个内含子组成,AGPS2a与AGPS2b的第一个外显子分别为1a与1b。
Fig. 6. Fine-mapping of WS gene. A, WS was linked with markers HY8-19 and HY8-24; B, WS was located in a 95 kb region based on 2025 individuals; C, Candidate genes for WS; D, ws displayed a single nucleotide substitution in AGPS2 (in red),AGPS2 is composed of 10 exons (filled box) and 9 introns, the alternative use of exon 1a and 1b generates AGPS2a and AGPS2b transcripts, respectively.
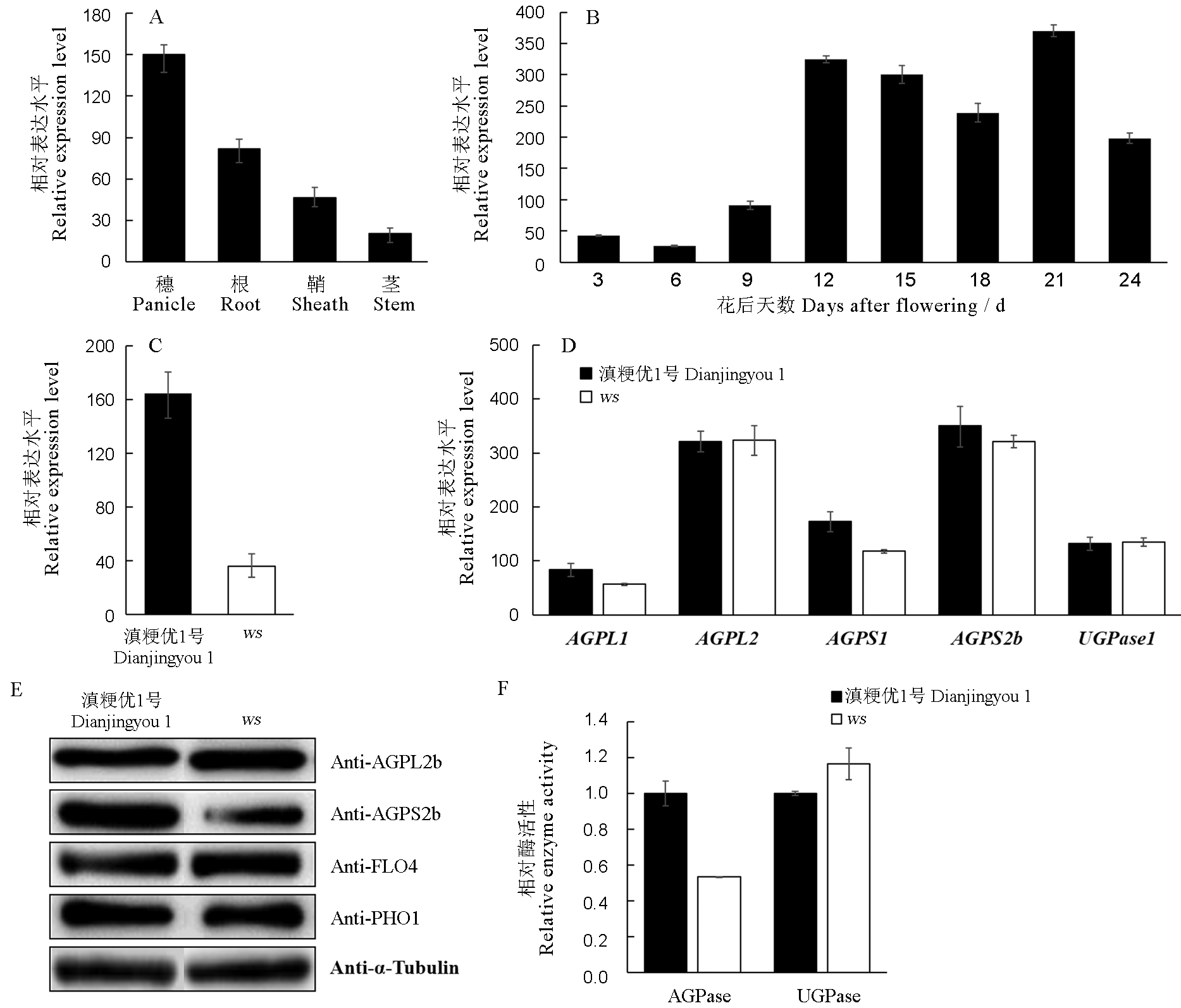
图7 野生型及ws突变体中的相关基因表达及酶活分析 A-野生型不同组织中AGPS2b的表达分析; B-野生型不同发育时期胚乳中AGPS2b的表达分析; C-野生型和ws突变体发芽7 d后幼苗叶片中AGPS2a表达分析; D-野生型与ws突变体发育中胚乳的AGPase相关基因表达分析; E-野生型与ws突变体发育中胚乳的相关蛋白表达分析; F-野生型和ws突变体发育胚乳中AGPase和UGPase活性测定。A,B,C,D,F中,n = 3,取平均值± SD。
Fig. 7. Expression of related genes and enzyme activity in wild type and ws mutant. A, Real-time RT-PCR analysis of the expression of AGPS2b in different organs in wild type; B, Real-time RT-PCR analysis of the expression of AGPS2b at different developing stages of endosperm in wild type; C, Real-time RT-PCR analysis of the expression of AGPS2a in leaves of the wild type and ws mutant seedlings at 7 days after germination; D, Real-time RT-PCR analysis of the expression of genes encoding AGPase in wild type and ws mutant endosperm; E, Immunoblot analysis of starch biosynthesis related proteins in wild type and ws mutant endosperm; F, AGPase and UGPase activities of wild-type and ws developing endosperm. For A, B, C, D and F, error bars show SD (n = 3).
| [1] | Buléon A, Colonna P, Planchot V, et al.Starch granules: Structure and biosynthesis.Int J Biol Macr, 1998, 23(2): 85-112. |
| [2] | Hirose T, Terao T.A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.) .Planta, 2004, 220(1): 9-16. |
| [3] | Ohdan T, Francisco P B, Sawada T, et al.Expression profiling of genes involved in starch synthesis in sink and source organs of rice.J Exp Bot, 2005, 56(422): 3229-3244. |
| [4] | Tetlow I J, Morell M K, Emes M J.Recent developments in understanding the regulation of starch metabolism in higher plants.J Exp Bot, 2004, 55(406): 2131-2145. |
| [5] | Fujita N, Satoh R, Hayashi A, et al.Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIa.J Exp Bot, 2011, 62(14): 4819-4831. |
| [6] | Hanashiro I, Itoh K, Kuratomi Y, et al.Granule-bound starch synthase I is responsible for biosynthesis of extra-long unit chains of amylopectin in rice.Plant & Cell Physiol, 2008, 49(6): 925-933. |
| [7] | Liu L, Ma X, Liu S, et al.Identification and characterization of a novel Waxy allele from a Yunnan rice landrace.Plant Mol Biol, 2009, 71(6): 609-626. |
| [8] | Nishi A, Nakamura Y, Tanaka N, et al.Biochemical and genetic analysis of the effects of Amylose-Extender mutation in rice endosperm.Plant Physiol, 2001, 127(2): 459-472. |
| [9] | Satoh H, Nishi A, Yamashita K, et al.Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm.Plant Physiol, 2003, 133(3): 1111-1121. |
| [10] | Kubo A, Fujita N, Harada K, et al.The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm.Plant Physiol, 1999, 121(2): 399-410. |
| [11] | Fujita N, Toyosawa Y Y, Higuchi T, et al.Characterization of pullulanase (PUL)-deficient mutants of rice (Oryza sativa L.) and the function of PUL on starch biosynthesis in the developing rice endosperm.J Nano Res, 2008, 16(1): 43-48. |
| [12] | Preiss J.Bacterial glycogen synthesis and its regulation.Ann Rev Microbiol, 1984, 38(1):419-458. |
| [13] | Villand P, Olsen O A, Kleczkowski L A.Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana.Plant Mol Biol, 1993, 23(6): 1279-1284. |
| [14] | Okita T W, Nakata P A, Anderson J M, et al.The subunit structure of potato tuber ADPglucose pyrophosphorylase.Plant Physiol, 1990, 93(2): 785-790. |
| [15] | Takashi A, Kouichi M, Tatsuhito F.Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA.Plant & Cell Physiol, 2005, 46(6): 937-946. |
| [16] | Binquan H, Hennen-Bierwagen T A, Myers A M. Functions of multiple genes encoding ADP-glucose pyrophosphorylase subunits in maize endosperm, embryo, and leaf.Plant Physiol, 2014, 164(2): 596-611. |
| [17] | Sikka V K, Choi S B, Kavakli I H, et al.Subcellular compartmentation and allosteric regulation of the rice endosperm ADP-glucose pyrophosphorylase.Plant Sci, 2001, 161(3):461-468. |
| [18] | Tetlow I J, Davies E J, Vardy K A, et al.Subcellular localization of ADP-glucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform.J Exp Bot, 2003, 54(383):715-725. |
| [19] | Kawagoe Y, Kubo A, Satoh H, et al.Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm.Plant J, 2005, 42(2): 164-174. |
| [20] | Lee S K, Hwang S K, Han M, et al.Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.).Plant Mol Biol, 2007, 65(4): 531-546. |
| [21] | Johnson P E, Patron N J, Bottrill A R, et al.A low-starch barley mutant, risø 16, lacking the cytosolic small subunit of ADP-glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit.Plant Physiol, 2003, 131(2):81-99. |
| [22] | Sakulsingharoj C, Choi S, Hwang S, et al.Engineering starch biosynthesis for increasing rice seed weight: The role of the cytoplasmic ADP-glucose pyrophosphorylase.Plant Sci, 2004, 167(6):1323-1333. |
| [23] | Curtis L H, Brandon F, James B, et al.A shrunken-2 transgene increases maize yield by acting in maternal tissues to increase the frequency of seed development.Plant Cell, 2012, 24(6):2352-2363. |
| [24] | Satoh H, Omura T.New endosperm mutations induced by chemical mutagens in rice Oryza sativa L.Jpn J Breeding, 1981, 31(3):316-326. |
| [25] | She K C, Kusano H, Koizumi K, et al.A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality.Plant Cell, 2010, 22(10): 3280-3294. |
| [26] | Nishio T, Iida S.Mutants having a low content of 16-kDa allergenic protein in rice (Oryza sativa L.).Theor Appl Genet, 1993, 86(2-3): 317-321. |
| [27] | Kang H G, Park S, Matsuoka M, et al.White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB).Plant J, 2005, 42(6): 901-911. |
| [28] | Ryoo N, Yu C, Park C S, et al.Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.).Plant Cell Rep, 2007, 26(7):1083-1095. |
| [29] | Peng C, Wang Y, Liu F, et al.FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm.Plant J, 2014, 77(6): 917-930. |
| [30] | Zhang L, Ren Y, Lu B, et al.FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice.J Exp Bot, 2015, 67(3):633-647. |
| [31] | Wu K S, Tanksley S D.Abundance, polymorphism and genetic mapping of microsatellites in rice.Mol General Genet, 1993, 241(1-2): 225-235. |
| [32] | 李永庚, 于振文, 姜东, 等. 冬小麦旗叶蔗糖和籽粒淀粉合成动态及与其有关的酶活性的研究. 作物学报, 2001, 27(5): 658-664. |
| Li Y G, Yu Z, Jiang D, et al.Studies on the dynamic changes of the synthesis of sucrose in the flag leaf and starch in the grain and related enzymes of high-yield wheat.Acta Agron Sin, 2001, 27(5): 658-664. (in Chinese with English abstract) | |
| [33] | Denyer K, Dunlap F, ThorbjoRnsen T, et al. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial.Plant Physiol, 1996, 112(2): 779-785. |
| [34] | Thorbjornsen T, Villand P, Denyer K, et al.Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm.Plant J, 1996, 10(2): 243-250 |
| [35] | Hannah L C, Shaw J R, Giroux M J, et al.Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase.Plant Physiol, 2001, 127(1): 173-183. |
| [36] | Sikka V K, Choi S B, Kavakli I H, et al.Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase.Plant Sci, 2001, 161(3): 461-468. |
| [37] | Seon-Kap H, Yasuko N, Dongwook K, et al.Direct appraisal of the potato tuber ADP-glucose pyrophosphorylase large subunit in enzyme function by study of a novel mutant form.J Biol Chem, 2008, 283(11): 6640-6647. |
| [38] | Laughlin M J, Chantler S E, Okita T W.N- and C-terminal peptide sequences are essential for enzyme assembly, allosteric, and/or catalytic properties of ADP-glucose pyrophosphorylase.Plant J, 1998, 14(2): 159-168. |
| [39] | Pedro C, Ballicora M A, Angel M, et al.The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme.J Biol Chem, 2003, 278(31): 28508-28515. |
| [40] | Petreikov M, Eisenstein M, Yeselson Y, et al.Characterization of the AGPase large subunit isoforms from tomato indicates that the recombinant L3 subunit is active as a monomer.Biochem J, 2010, 428(2): 201-212. |
| [41] | Ventriglia T, Kuhn M L, Ruiz M T, et al.Two Arabidopsis ADP-glucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic.Plant Physiol, 2008, 148(1): 65-76. |
| [42] | Aytug T, Joe K, Yasuharu I, et al.The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme.Plant Cell Physiol, 2014, 55(6): 1169-1183. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 肖正午, 方升亮, 曹威, 胡丽琴, 黎星, 解嘉鑫, 廖成静, 康玉灵, 胡玉萍, 张珂骞, 曹放波, 陈佳娜, 黄敏. 米粉质构特性与稻米理化性状的关系[J]. 中国水稻科学, 2024, 38(3): 316-323. |
| [14] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [15] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||