
中国水稻科学 ›› 2015, Vol. 29 ›› Issue (1): 35-44.DOI: 10.3969/j.issn.1001-7216.2015.01.005
周红敏, 王化敦, 孙瑞, 裴文霞, 吴学能, 曹越, 孙雅菲, 徐国华, 孙淑斌*( )
)
收稿日期:2014-01-09
修回日期:2014-03-08
出版日期:2015-01-10
发布日期:2015-01-10
通讯作者:
孙淑斌
基金资助:
Hong-min ZHOU, Hua-dun WANG, Rui SUN, Wen-xia PEI, Xue-neng WU, Yue CAO, Ya-fei SUN, Guo-hua XU, Shu-bin SUN*( )
)
Received:2014-01-09
Revised:2014-03-08
Online:2015-01-10
Published:2015-01-10
Contact:
Shu-bin SUN
摘要:
植物应对磷胁迫的方法之一是改变根系的构型。以水稻SUMO化E3连接酶SIZ1突变体ossiz1为供试材料,研究了OsSIZ1在水稻根发育中的作用以及其与磷胁迫、生长素之间的关系。与野生型相比,OsSIZ1抑制ossiz1种子根和不定根的伸长,促进侧根密度的增加和根毛的增多。缺磷时,突变体ossiz1的反应更强烈,即不定根伸长、侧根密度增大和根毛增多的趋势更加明显。说明OsSIZ1参与调控水稻根构型的改变,低磷时效果更明显。ossiz1地上部和地下部的总磷浓度显著高于野生型,说明OsSIZ1在水稻中负调控磷素的吸收利用。定量RT-PCR结果显示,ossiz1中OsYUCCA1和OsPIN1a/1b的相对表达量显著高于野生型,说明OsSIZ1负调控根中生长素的合成与极性运输,并且缺磷时负调控作用减弱。结果表明,SUMO化E3连接酶OsSIZ1调控缺磷条件下根构型的形成,而且这一过程可能是通过调控生长素分布完成的。
中图分类号:
周红敏, 王化敦, 孙瑞, 裴文霞, 吴学能, 曹越, 孙雅菲, 徐国华, 孙淑斌. 水稻SUMO化E3连接酶SIZ1调控缺磷条件下根的发育和根构型形成[J]. 中国水稻科学, 2015, 29(1): 35-44.
Hong-min ZHOU, Hua-dun WANG, Rui SUN, Wen-xia PEI, Xue-neng WU, Yue CAO, Ya-fei SUN, Guo-hua XU, Shu-bin SUN. OsSIZ1 Regulates the Development and Architecture of the Roots under Phosphate Starvation Conditions in Rice[J]. Chinese Journal OF Rice Science, 2015, 29(1): 35-44.
| 基因 Gene | 引物序列 Primer sequences (5'-3') |
|---|---|
| OsActin | F: GGAACTGGTATGGTCAAGGC |
| R: AGTCTCATGGATAACCGCAG | |
| OsPIN1a | F: TCATCTGGTCGCTCGTCTGC |
| R: CGAACGTCGCCACCTTGTTC | |
| OsPIN1b | F: TGCACCCTAGCATTCTCAGCA |
| R: CCCTCCTCCCAAATTCTACTT | |
| OsYUCCA1 | F: TCATCGGACGCCCTCAACGTCGC |
| R: GGCAGAGCAAGATTATCAGTC | |
| OsPIN2 | F:CAACACCTACTCCAGCCTC |
| R:TGGACCAGTCAAGAACCTC |
表1 生长素生物合成基因OsYUCCA1和生长素外流蛋白基因OsPINs的序列
Table 1 Primers used to amplify the OsYUCCA1 and OsPINs cDNA fragments.
| 基因 Gene | 引物序列 Primer sequences (5'-3') |
|---|---|
| OsActin | F: GGAACTGGTATGGTCAAGGC |
| R: AGTCTCATGGATAACCGCAG | |
| OsPIN1a | F: TCATCTGGTCGCTCGTCTGC |
| R: CGAACGTCGCCACCTTGTTC | |
| OsPIN1b | F: TGCACCCTAGCATTCTCAGCA |
| R: CCCTCCTCCCAAATTCTACTT | |
| OsYUCCA1 | F: TCATCGGACGCCCTCAACGTCGC |
| R: GGCAGAGCAAGATTATCAGTC | |
| OsPIN2 | F:CAACACCTACTCCAGCCTC |
| R:TGGACCAGTCAAGAACCTC |
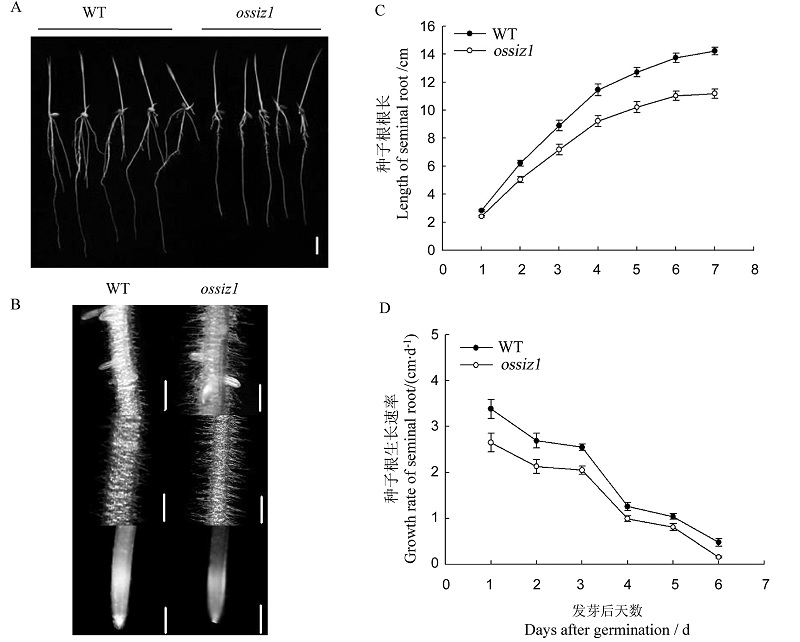
图1 突变体ossiz1及其野生型种子根的生长情况 A-发芽7 d后水稻幼苗种子根的表型,白色线条表示1 cm; B-发芽7 d后水稻幼苗种子根不同部位的根毛生长,白色线条表示2 cm; C和D-营养液开始处理后7 d内水稻幼苗种子根的根长和生长速率。WT-野生型。
Fig.1. Growth performances of seminal roots of ossiz1 and wild type. A, Root phenotype of rice seedlings 7 d after germination, bar=1 cm; B, The root hair proliferation on the seminal roots of rice seedlings 7 d after germination, bar=2 cm; C,D, The length and the growth rate of seminal root of rice seedlings for 7 d after transfer to the nutrient solution. WT, Wild type.
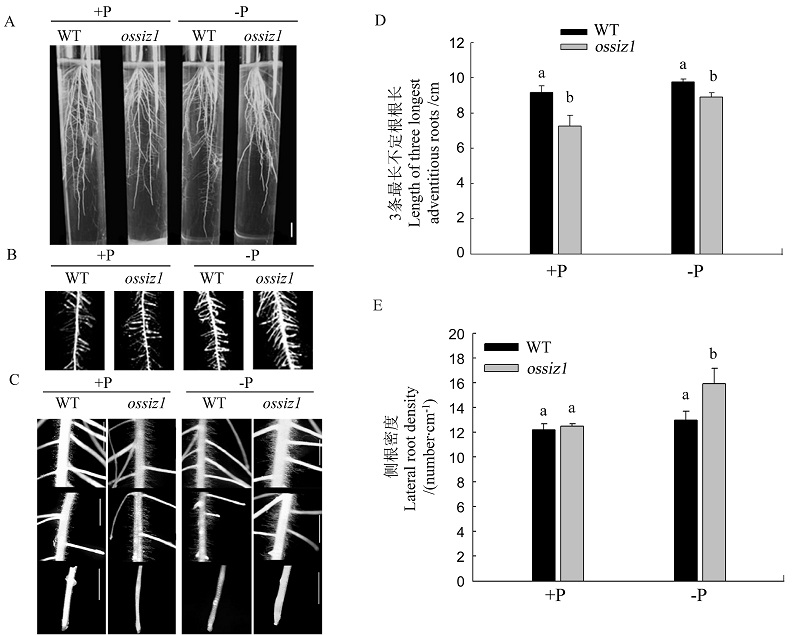
图2 正常供磷(+P)和缺磷(-P)固体培养基中野生型和ossiz1根系的表型分析 A、C中白色线条分别表示1 cm,2 cm。
Fig. 2. Root phenotypic analysis of wild type(WT) and ossiz1 seedlings in the Pi-sufficient(+P) and Pi-deficient(-P) solid culture medium. In A, Bar=1 cm; In C, Bar=2 cm.
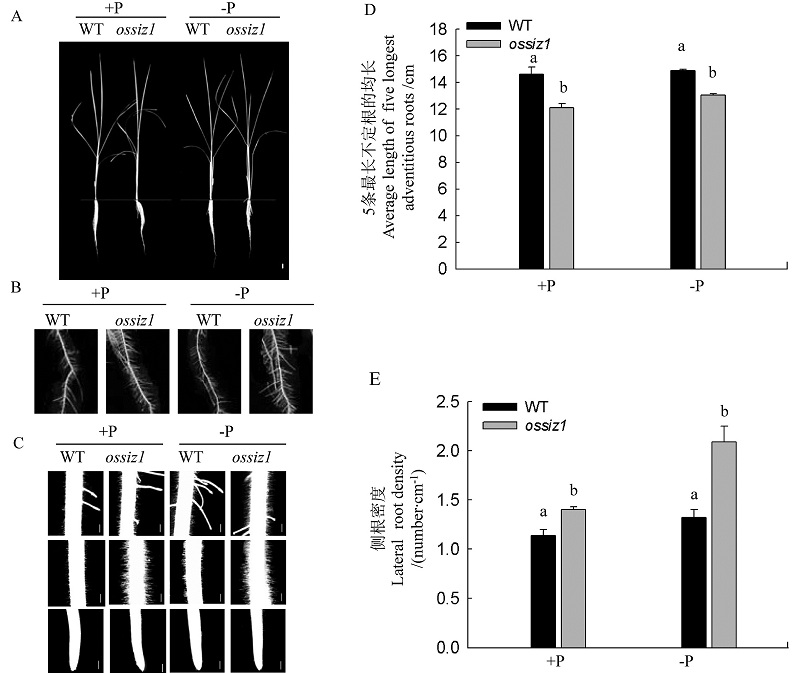
图3 正常供磷(+P)和缺磷(-P)条件下ossiz1突变体及其野生型根系的表型分析 A、C中白色线条分别表示1 cm; 2 cm。
Fig. 3. Root phenotype of wild type (WT) and ossiz1 seedlings under Pi-sufficient(+P) and Pi-deficient(-P) conditions. In A, Bar=1 cm; In C; Bar=2 cm.
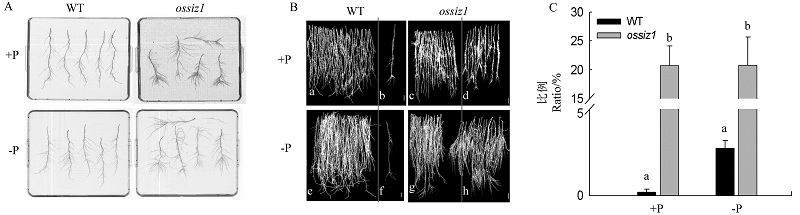
图4 正常供磷和缺磷条件下ossiz1突变体及其野生型根系中不定根的统计分析 A-不定根的扫描图; B-每株水稻苗根系中无成簇大侧根发生的不定根(a, c, e, g)和有成簇大侧根发生的不定根(b, d, f, h); C-有成簇大侧根发生的不定根占总不定根数的百分比。
Fig. 4. Statistical analysis of adventitious roots of ossiz1 and its wild type (WT) seedlings under Pi-sufficient (+P) and Pi-deficient (-P) conditions. A,Root scanning; B, Adventitious roots without large lateral root formation in clumps (a, c, e, g) and adventitious roots with large lateral root formation in clumps (b, d, f, h); C,The ratio of adventitious roots with large lateral root formation in clumps to the total adventitious roots.
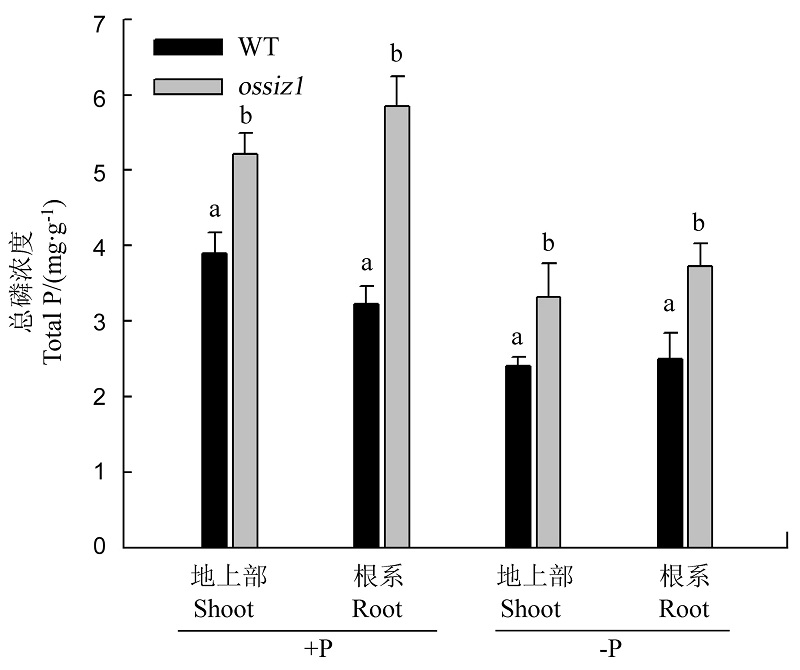
图5 水培正常供磷和缺磷条件下ossiz1及其野生型地上部和地下部总磷浓度
Fig. 5. Total P concentration in the shoots and roots of ossiz1 and its wild type (WT) seedlings under Pi-sufficient (+P) and Pi-deficient (-P) conditions in hydroponics culture.
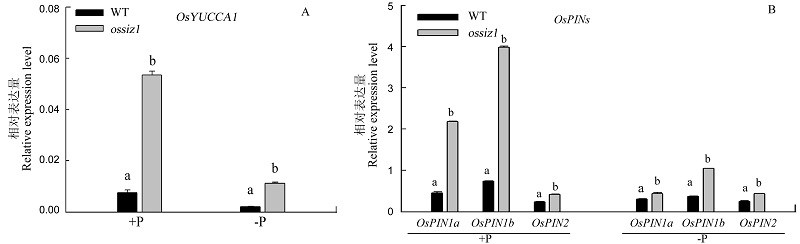
图6 水培试验时WT和ossiz1根中生长素途径中相关基因的表达 A-正常供磷和缺磷处理2周后生长素生物合成基因OsYUCCA1的相对表达量; B-正常供磷和缺磷处理2周后生长素外流基因OsPINs的相对表达量。
Fig. 6. Expression of auxin genes in roots of WT and ossiz1 in hydroponics experiment. A,Relative expression of auxin biosynthesis gene OsYUCCA1 subjected to +P and -P conditions for 2 weeks; B, Relative expression of auxin efflux transporters OsPINs subjected to +P and -P conditions for 2 weeks.
| [1] | 杨辉霞, 童依平, 王道文. 拟南芥低磷胁迫反应分子机理研究的最新进展. 植物学通报, 2007, 24: 726-734. |
| [2] | Chiou T J, Lin S I.Signaling network in sensing phosphate availability in plants.Annu Rev Plant Biol, 2011, 62: 185-206. |
| [3] | Grime J P, Crick J C, Rincon J E.The ecological significance of plasticity.Symp Soc Exp Biol, 1986, 40: 5-29. |
| [4] | Price A H, Tomos A D, Virk D S.Genetic dissection of root growth in rice (Oryza sativa L.): Ⅰ. A hydrophonic screen.Theor Appl Genet, 1997, 95: 132-142. |
| [5] | Narang R A, Bruene A, Altmann T.Analysis of phosphate acquisition efficiency in different Arabidopsis accessions.Plant Physiol, 2000, 124: 1786-1799. |
| [6] | Malamy J E.Intrinsic and environmental response pathways that regulate root system architecture.Plant Cell Environ, 2005, 28: 67-77. |
| [7] | López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L.The role of nutrient availability in regulating root architecture.Curr Opin Plant Biol, 2003, 6: 280-287. |
| [8] | Raghothanma K G.Phosphate acquisition.Annu Rev Plant Physiol Plant Mol Biol, 1999, 50: 665-693. |
| [9] | 黄荣, 孙虎威, 刘尚俊, 等. 低磷胁迫下水稻根系的发生及生长素的响应. 中国水稻科学, 2012, 26(5): 563-568. |
| [10] | Friml J, Vieten A, Sauer M, et al.Efflux-dependent auxin gradients establish the apical-basal axis ofArabidopsis. Nature, 2003, 426: 47-53. |
| [11] | Benkova E, Michniewicz M, Sauer M, et al.Local, efflux-dependent auxin gradients as a common module for plant organ formation.Cell, 2003, 115: 591-602. |
| [12] | Mattsson J, Ckurshumova W, Berleth T.Auxin signaling in Arabidopsis leaf vascular development.Plant Physiol, 2003, 131: 1327-1339. |
| [13] | Blakeslee J J, Bandyopadhyay A, Peer W A, et al.Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses.Plant Physiol, 2004, 134: 28-31. |
| [14] | Palme K, Dovzhenko A, Ditengou F A.Auxin transport and gravitational research: Perspectives.Protoplasma, 2006, 229: 175-181. |
| [15] | Vanneste S, Friml J.Auxin: A trigger for change in plant development.Cell, 2009, 136: 1005-1016. |
| [16] | Zhi G E, Ge L, Wang L.Molecular mechanism of adventitious root formation in rice.Plant Growth Regul, 2012, 68: 325-331. |
| [17] | Yamamoto Y, Kamiya N, Morinaka Y, et al.Auxin biosynthesis by the YUCCA genes in rice.Plant Physiol, 2007, 143: 1362-1371. |
| [18] | Morita Y, Kyozuka J.Characterization of OsPID, the rice ortholog of PINOID, and its possible involvement in the control of polar auxin transport.Plant Cell Physiol, 2007, 48: 540-549. |
| [19] | Dong L, Wang L, Zhang Y, et al.An auxin-inducible F-box protein CEGENDUO negatively regulates auxin-mediated lateral root formation in Arabidopsis.Plant Mol Biol, 2006, 60: 599-615. |
| [20] | Lee S H, Cho H T.PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells.Plant Cell, 2006, 18: 1604-1616. |
| [21] | Duan Q H, Kita D, Li C, et al.FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development.PNAS, 2010, 107(41): 17821-17826. |
| [22] | Shen C J, Wang S K, Zhang S N, et al.OsARF16, a transcription factor, is required for auxin and phosphate starvation response in rice (Oryza sativa L.). Plant, Cell,Environ, 2013, 36: 607-620. |
| [23] | Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, et al.An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis.Plant Physiol, 2005, 137: 681-691. |
| [24] | Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, et al.Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system.Plant Physiol, 2002, 129: 244-256. |
| [25] | Pérez-Torres C A, Lo’pez-Bucio J, Cruz-Ramı’rez A, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor.Plant Cell, 2008, 20: 3258-3272. |
| [26] | Nacry P, Canivenc G, Muller B, et al.A role for auxin redistribution in the responses of the root system architecture to phosphate starvation inArabidopsis. Plant Physiol, 2005, 138: 2061-2074. |
| [27] | Chen Y N, Fan X R, Song W J, et al.Over-experssion of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1.Plant Biol, 2012, 10: 139-149. |
| [28] | 陈泉, 施蕴渝. 小泛素相关修饰SUMO研究进展. 生命科学, 2004, 16(1): 1-6. |
| [29] | 徐庞连, 曾棉炜, 黄丽霞,等. 植物SUMO化修饰及其生物学功能.植物学通报, 2008, 25(5): 608-615. |
| [30] | Miura K, Rus A, Sharkhuu A, et al.Arabidopsis SUMO E3 ligase SIZl controls phosphate deficiency responses.PNAS, 2005, 102: 7760-7765. |
| [31] | Miura K, Jin J B, Hasegawa P M.Sumoylation, a post-translational regulatory process in plants.Curr Opin Plant Biol, 2007, 10: 495-502. |
| [32] | Yoo C Y, Miura K, Jin J B, et al.SIZl small ubiquitin-like modifier E3 ligase facilitates basal thermotolerance in Arabidopsis independent of salicylic acid.Plant Physiol, 2006, 142: 1548-1558. |
| [33] | Catala R, Ouyang J, Abreu I A, et al.The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses.Plant Cell, 2007, 19: 2952-2966. |
| [34] | Saminathan T, Guo C L, Chuang M H.Rice SIZ1, a SUMO E3 ligase, controls spikelet fertility through regulation of anther dehiscence.New Phytol, 2011, 189(3): 869-882. |
| [35] | Miura K, Rus A, Sharkhuu A, et al.The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses.PNAS, 2005, 102: 7760-7765. |
| [36] | Miura K, Lee J, Gong Q.SIZ1 Regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation.Plant Physiol, 2011, 155: 1000-1012. |
| [37] | Wang H D, Makeen K, Yan Y.OsSIZ1 regulates the vegetative growth and reproductive development in rice.Plant Mol Biol Rep, 2010, 29: 411-417. |
| [38] | 鲍士旦. 土壤农化分析.北京:中国农业出版社, 1999. |
| [39] | Beemster G T, De Vusser K, De Tavernier E, et al.Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-typecyclin-dependent kinase activity.Plant Physiol, 2002, 129: 854-864. |
| [40] | Mouchel C F, Briggs G C, Hardtke C S.Natural genetic variation in Arabidopsis identifies BREVIS RADIX, a novel regulator of cell proliferation and elongation in the root.Genes Dev, 2004, 18: 700-714. |
| [41] | Zhu Y, Dong A, Meyer D, et al.Arabidopsis NRP1 and NRP2 encode histone chaperones and are required for maintaining postembryonic root growth.Plant Cell, 2006, 18: 2879-2892. |
| [42] | Jia L, Zhang B, Mao C, et al.OsCYT-INV1 for alkaline/neutral invertase is involved in root cell development and reproductivity in rice (Oryza sativa L.).Planta, 2008, 228: 51-59. |
| [43] | Miura K, Lee J, Miura T, Hasegawa PM.SIZ1 controls cell growth and plant development in Arabidopsis through salicylic acid.Plant Cell Physiol, 2010, 51: 103-113. |
| [44] | Huang L, Yang S, Zhang S, et al.The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root.Plant J, 2009, 60: 666-678. |
| [45] | 郭朝晖, 张杨珠, 黄见良, 等.磷对杂交水稻根系特性与养分吸收的影响. 湖南农业大学学报.自然科学版, 2001, 27(5):350-354. |
| [46] | 郭再华, 贺立源, 徐才国.水稻耐低磷特性研究.应用与环境生物学报, 2004, 10(6):681-685. |
| [47] | 郭再华, 贺立源, 徐才国.磷水平对不同耐低磷水稻苗根系生长及氮、磷、钾吸收的影响. 应用与环境生物学报, 2006, 12(4):449-452. |
| [48] | Sasaki O, Yamazaki K, Kawata S.The relationship between the diameters and the structures of lateral roots in rice plants.Jpn J Crop Sci, 1984, 53: 169-175. |
| [49] | Rebouillat J, Dievart A, Verdeil J L.Molecular genetics of rice root development.Rice, 2009, 2: 15-34. |
| [50] | Zhu Z X, Liu Y, Liu S J, et al.A gain-of-function mutation in OsIAA11 affects lateral root development in rice.Mol Plant, 2012, 5: 154-161. |
| [51] | Grierson C, Schiefelbein J.Root Hairs// The Arabidopsis Book. Roekville MD: Ameriean Society of Plant Biologists, 2002. |
| [52] | Peret B, De Rybel B, Casimiro I, et al.Arabidopsis lateral root development: An emerging story.Trends Plant Sci, 2009, 14: 399-408. |
| [53] | Benkova E, Bielach A.Lateral root organogenesis-from cell to organ.Curr Opin Plant Biol, 2010, 13: 677-683. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||